INTRODUCTION
Patients in the intensive care unit (ICU) who experience delirium are exhibiting an under-recognized form of organ dysfunction. Delirium is extremely common in ICU patients as factors such as comorbidity, the acute critical illness itself, and iatrogenesis intersect to create a high-risk setting for delirium. This neurologic complication is often hazardous, being associated with death, prolonged hospital stays, and long-term cognitive impairment and institutionalization. Neurologic dysfunction compromises patients’ ability to be removed from mechanical ventilation or to fully recover and regain independence. Unfortunately, health care providers in the ICU are unaware of delirium in many circumstances, especially those in which the patient’s delirium is manifesting predominantly as the hypoactive (quiet) subtype rather than the hyperactive (agitated) subtype. Despite being often overlooked clinically, ICU delirium has increasingly been the subject of research during the past decade, which has brought to light the scope of the problem in critically ill patients and provided clinicians with tools for routinely monitoring delirium at the bedside. This chapter reviews the definition and salient features of delirium, its primary risk factors, including drugs associated with the development of delirium, proposed pathophysiologic mechanisms, validated methods for bedside delirium assessment, and nonpharmacologic and pharmacologic strategies for delirium management.
DEFINITION AND TERMINOLOGY
The American Psychological Association’s (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV describes delirium as a disturbance in consciousness and cognition that develops over a short period of time (eg, hours to days) and tends to fluctuate during the course of the day.1 Specifically, there are four criteria required to diagnose delirium1:
Disturbance of consciousness, with reduced awareness of the environment and impaired ability to focus, sustain or shift attention.
Altered cognition (eg, memory impairment, disorientation, or language disturbance) or the development of a perceptual disturbance (eg, delusion, hallucination, or illusion) that is not better accounted for by preexisting or evolving dementia.
Disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day.
Evidence of an etiological cause, which the DSM-IV uses to classify delirium as Delirium Due to a General Medical Condition, Substance-Induced Delirium, Delirium Due to Multiple Etiologies, or Delirium Not Otherwise Specified.
Historically, two words were used to describe acutely confused patients. The Roman word delirium referred to an agitated and confused person (ie, hyperactive delirium). The Greek word lethargus was used to describe a quietly confused person (ie, hypoactive delirium). ICU patients commonly demonstrate both subtypes of delirium as they progress through different stages of their illness and therapy. In both subtypes, the patient’s brain is not functioning normally. It therefore makes sense that the original derivation of delirium comes from the Latin word deliria, which literally means to “be out of your furrow.” For greater clarity and to avoid misuse of terms such as dementia and delirium, Table 82-1 lists basic definitions and clinical characteristics of each syndrome.
Differentiating Delirium From Dementia
| Delirium | Dementia | |
|---|---|---|
| Onset | Acute (hours to days) | Insidious (months to years) |
| Course | Fluctuating | Progressive |
| Diagnostic Features |
|
|
| Associated Features |
|
|
| Common Causes |
|
|
Delirium in the ICU has been referred to in the medical literature using a multitude of terms, including ICU psychosis, ICU syndrome, brain failure, encephalopathy, postoperative psychosis, acute organic syndrome, subacute befuddlement, and toxic confusional state.2-5 Neurologists often use “encephalopathy” to refer to hypoactive delirium and “delirium” to describe only hyperactive delirium.6 Among ICU practitioners, “delirium” is used inconsistently, as evidenced by a recent survey of Canadian intensivists that found respondents were more likely to use the term “delirium” when no specific underlying etiology could be identified for a patient with fluctuating mental status with inattention, perceptual changes, and disorganized thinking, whereas alternative terms (eg, hepatic encephalopathy) were used when the etiology of delirium was obvious.5,7
Increasingly, however, the ICU community is seeking to standardize delirium terminology to conform to the APA definition, with the hope that use of “delirium” to describe this syndrome of acute brain dysfunction, regardless of etiology, will improve cross-talk between specialists with different medical backgrounds, collaborative research efforts, and ultimately management of this widely prevalent syndrome.4 Therefore, the unifying term “delirium” should be applied whenever patients meet DSM-IV diagnostic criteria for delirium, and the underlying etiology, when known, can be used as an associated term (eg, “delirium secondary to sepsis” is preferred over “septic encephalopathy”).
PREVALENCE AND SUBTYPES
Delirium during critical illness occurs in 20% to 80% of ICU patients depending on the severity of illness of the population studied and methods used to detect delirium.8-16 The prevalence is highest, for example, in mechanically ventilated ICU patients, with 60% to 80% developing delirium during their ICU stay,8,10,12,14,17 whereas lower prevalence rates are reported in nonventilated patients and in mixed ICU populations.9,11,18 In general, ICU patients have a higher prevalence of delirium compared with noncritically ill hospitalized patients.19,20 The prevalence of ICU delirium will likely increase as the U.S. population ages.
Delirium can be subtyped based on observed changes in motor activity, resulting in hypoactive, hyperactive, and mixed subtypes.21 Peterson et al reported these delirium subtypes in a cohort of 613 ventilated and nonventilated ICU patients in whom delirium was monitored for more than 20,000 observations. Among patients who developed delirium, pure hyperactive delirium was rare (<5%), whereas hypoactive was present in 45% and the mixed subtype—with alternating periods of hypoactive and hyperactive delirium—was the predominant manifestation (54%). Interestingly, hypoactive delirium was significantly more common in patients over the age of 65. Similarly, in a cohort of 100 surgical and trauma ICU patients, the prevalence of hypoactive delirium was greater than 60%.22 The risk factors for, and clinical implications of, these subtypes are the subject of ongoing investigations.23
Because sedation is commonly used in the ICU, the period surrounding cessation of sedation represents a scenario in the ICU during which delirium could be easily recognized but is often missed. Delirious patients emerging from the effects of sedation may do so peacefully or in a combative manner. The “peaceful” patients are often erroneously assumed to be thinking clearly. Delirium in this context is referred to as hypoactive delirium and is characterized by lethargy, drowsiness, and infrequent spontaneous movement,21 which contributes to delirium being overlooked unless the patient is specifically screened for its presence.24-28 Even in the absence of agitation, such delirium can lead to adverse outcomes such as reintubation, which itself has been shown to increase the risk of prolonging the ICU stay, transfer to a long-term care or rehabilitation facility, and death.29 In addition, hypoactive delirium is associated with immobility in the ICU,30 which itself places patients at risk for adverse outcomes, including aspiration, pulmonary embolism, and decubitus ulcers.
In contrast to patients with hypoactive delirium are agitated or combative patients with hyperactive delirium; these patients are at risk not only for self-extubation and subsequent reintubation but also for pulling out central venous catheters and even falling out of bed. These hyperactive patients are often given large doses of sedatives that lead to heavy sedation and prevent timely liberation from mechanical ventilation, placing patients at risk for remaining delirious or even comatose and on invasive mechanical ventilation unnecessarily.31 To avoid this difficult and dangerous cycle, health care professionals should minimize use of psychoactive medications and frequently assess patients for delirium, especially during the transition from drug-induced or metabolic coma to wakefulness.
RISK FACTORS
Nearly every ICU patient is exposed to one or more risk factors for delirium; the average patient in one study, in fact, had 11 identifiable risk factors for delirium.32 These risk factors may be divided into predisposing (baseline) factors and precipitating (hospitalization-related) factors.33 Patients who are highly vulnerable to developing delirium (ie, who have multiple predisposing risk factors) may become delirious with only minor insults, whereas those with low baseline vulnerability may require a greater insult to become delirious.33 Predisposing risk factors, those related to patient characteristics or underlying chronic pathology, are difficult to alter, whereas precipitating factors, such as those related to the acute illness or the ICU environment, represent areas of risk that are modifiable or preventable (Table 82-2).
Risk Factors for Delirium
| Host Factors | Factors Relating to Critical Illness | Environmental and Iatrogenic | |
|---|---|---|---|
| Not modifiable or preventable |
|
|
|
| Potentially modifiable/preventable |
|
|
|
Baseline risk factors that have been identified in both ICU and non-ICU populations include older age, depression, vision impairment, hearing impairment, hypertension, history of smoking, history of alcohol use, living single at home, underlying cognitive impairment or dementia, and APOE4 polymorphism.9,10,13,34-37 Numerous features of the acute critical illness have been identified as delirium risk factors in studies specifically examining ICU patients; these include admission to an ICU for a medical illness, high severity of illness (indicated by high APACHE II and SAPS II scores), need for mechanical ventilation, receipt of sedative and/or analgesic medications (particularly when used to induce coma), respiratory disease, anemia, hypotension, hypocalcemia, hyponatremia, azotemia, transaminitis, hyperamylasemia, hyperbilirubinemia, acidosis, fever, infection, sepsis, gastric tubes, bladder catheters, arterial lines, and more than three infusing medications.9,13,17,35-39 Risk factors related to the ICU environment include lack of daylight in the ICU, isolation, lack of visitors, and sleep disturbances.37,40
Though difficult to accurately measure in ICU patients, sleep deprivation is believed to be nearly universal in the ICU and has long been proposed as a risk factor for delirium. The relationship, however, between sleep disturbance and delirium in the ICU remains controversial, and there is significant overlap in the symptoms of both syndromes such that either may present with inattention, fluctuating mental status and cognitive dysfunction, making it difficult to ascertain whether sleep deprivation causes delirium or vice versa.40,41 On average, ICU patients sleep between 2 and 8 hours in a 24-hour period, often with severe and frequent disruptions and only a small fraction of “restorative,” rapid eye movement (REM) sleep.42 In repeated studies, between one-third and one-half of patients’ sleep in the ICU occurs during daytime hours.42,43 Reasons for poor sleep in this setting are multifactorial. The ICU environment, with its continuous cycle of alarms, lights, and care-related interruptions interferes with a patient’s sleep cycle and may disrupt their circadian rhythm.41,43 Acute illness, with symptoms such as nausea, pain, and fever, may also disrupt sleep. Mechanically ventilated patients may additionally suffer sleep disruptions due to anxiety, ventilator dyssynchrony, central apneas, and mode of mechanical ventilation.44 Finally, medications commonly given to ICU patients, such as sedatives, analgesics, vasopressors, β-agonists, and corticosteroids, disrupt slow-wave and REM sleep.45 Further study of sleep in the ICU is necessary to understand the underlying mechanisms for sleep disruption and the relationship between sleep and delirium. Meanwhile, clinicians should attend to modifiable risk factors by reducing noise and light at night, minimizing other disruptions in the ICU environment, treating symptoms, and judiciously using sleep-disrupting medications.
The deliriogenic effects of medications given for sedation and/or analgesia—drugs used to treat nearly all ICU patients at some time during their ICU stay—have received specific attention in many studies, as they represent a potent yet potentially modifiable risk factor for delirium. Though sedative and analgesic medications are prescribed to relieve pain and anxiety and to improve patient tolerance of treatments during critical illness, these medications have important side effects. Continuous infusion of sedatives, for example, is associated with prolonged mechanical ventilation,31 whereas interruption of sedative infusions expedites weaning from mechanical ventilation, speeds discharge from the ICU and hospital, and improves long-term survival.12,46
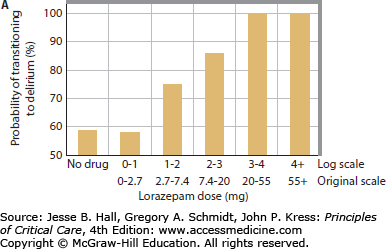
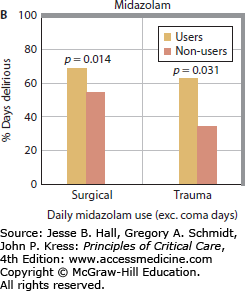
Multiple studies have now clearly demonstrated a link between benzodiazepines and development of delirium. Lorazepam dose was found to be an independent risk factor for the delirium in medical ICU patients, such that each day a patient was treated with the drug, the odds of being delirious the next day increased by 20%. In fact, patients treated with greater than 20 mg of lorazepam in a day were nearly all delirious or comatose the following day.13 Numerous other studies have consistently found similar links between benzodiazepine administration (whether lorazepam or midazolam) and delirium in patients in surgical, trauma, burn, and mixed ICUs (Fig. 82-1).14,15,17,36,38,39,47
FIGURE 82-1
Relationship between benzodiazepines and delirium. Multiple studies have demonstrated the association between benzodiazepines and delirium. As the daily dose of lorazepam increased in medical ICU patients, the odds of transitioning to delirium increase, such that patients treated with >20 mg of lorazepam per day universally developed delirium (A). Reproduced with permission from Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;(12 suppl 3):S3. Similarly, daily midazolam use is associated with an increase in the proportion of days with delirium in surgical and trauma ICU patients (B). Reproduced with permission from Pandharipande P, Cotton BA, Shintani A. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. July 2008;65(1):34-41.
Narcotic pain medications present a more complex picture in terms of their relationship with delirium in the ICU, in that they have been associated with development of delirium in some studies but not in others. This is likely due to the differing indications for (or dual effects of) analgesics in the ICU. Narcotic pain medications are associated with the development of delirium in populations frequently sedated with these drugs, such as medical and surgical ICU patients.9,17,37 In these settings, narcotics are often co-administered with benzodiazepines; in one study, elderly ICU patients treated with benzodiazepines and opioids had a longer duration of delirium.39 When narcotic medications are used to induce coma, the odds of developing delirium triple.36 Thus, clinicians should seek to minimize the use of heavily sedating medications, whether benzodiazepines or narcotics, by using evidenced based protocols to interrupt continuous sedative infusions12,46 and seek to use nonbenzodiazepine sedative medications where possible.14,15,48 Patients more often treated with narcotics because of pain, such as trauma ICU patients, are found to have a lower risk of the development of delirium when treated with fentanyl or morphine compared to patients who were not exposed to these drugs.17 Intravenous opiates and exposure to methadone was protective against development of delirium in burn ICU patients.47
PATHOPHYSIOLOGY
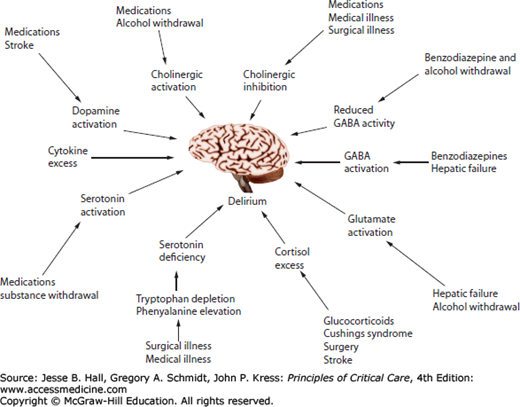
The pathophysiology of delirium remains incompletely understood. Leading hypotheses, often drawn from research outside the ICU, propose that delirium results from neurotransmitter imbalances and/or factors that affect neurotransmitter production, such as availability of large neutral amino acids, or systemic and central nervous system (CNS) inflammation. Delirium during critical illness is most likely a consequence of a complementary and interlinked series of events (Fig. 82-2).
FIGURE 82-2
Delirium pathophysiology represents a complex series of interrelated events. Multiple pathways to delirium may be present in a single patient. (Reproduced with permission from Flacker JM, Lipsitz LA, et al. Neural mechanisms of delirium: current hypotheses and evolving concepts. J Gerontol A Biol Sci Med Sci. June 1999;54(6):B239-B246.)
Delirium due to Atropa belladonna (a plant known as Deadly Nightshade, which contains the anticholinergic atropine) and anticholinergic drugs, such as scopolamine, has been recognized for centuries, an observation that led to the hypothesis that imbalances in the synthesis, release, and inactivation of neurotransmitters—especially acetylcholine and dopamine—that control arousal and the sleep-wake cycle are the underlying mechanism leading to delirium.49,50 Studies measuring the amount of anticholinergic activity in hospitalized patients found higher levels of serum anticholinergic activity (SAA) were associated with an increased risk of delirium, even in patients not exposed to medications with anticholinergic properties.51,52 Central cholinergic deficiency can theoretically result from derangements occurring anywhere along the continuum from acetylcholine production and release to its action on postsynaptic receptors. In addition to cholinergic deficiency, dopamine excess is thought to be associated with delirium, likely via its action on central dopamine receptors that regulate acetylcholine production.50-54 Finally, imbalances in the production, release, and degradation of numerous other neurotransmitters, such as serotonin, norepinephrine, glutamate, melatonin, and gamma-aminobutyric acid (GABA), have also been suspected to play a role in the development of delirium.49-54
Large neutral amino acids (LNAAs), including leucine, valine, tryptophan, tyrosine, and phenylalanine, are the precursors of several neurotransmitters that are involved in arousal, attention, and cognition and are therefore hypothesized to be involved in the pathogenesis of delirium.52 The synthesis of serotonin and melatonin depend on the availability of tryptophan, whereas the production of norepinephrine and dopamine require both tyrosine and phenylalanine. The LNAAs compete for transfer across the blood-brain barrier, such that an increase in one LNAA causes a decrease in the entry of other LNAAs into the brain.52 Thus, changes in serum levels of individual LNAAs may directly effect CNS neurotransmitter concentrations. With this in mind, Flacker and collegues55 examined LNAA levels in acutely ill elderly medical patients and found an association between delirium and an elevated plasma phenylalanine/LNAA ratio. Tryptophan/LNAA ratios are decreased and phenylalanine/LNAA ratios increased in cardiac surgery patients who developed delirium.56 Low plasma levels of tryptophan were also observed in delirious postoperative patients.57 Finally, Pandharipande and collaborators described both high and low tryptophan/LNAA ratios and high and low tyrosine/LNAA ratios as independent risk factors for delirium (with mid-range ratios being low-risk for delirium) in a cohort of mechanically ventilated ICU patients.58 These studies suggest that changes in LNAA concentrations with subsequent alterations in CNS neurotransmitter levels are important in the pathogenesis of delirium.
Delirium is also hypothesized to result from systemic inflammation, which occurs frequently in critical illness as a result of infection, tissue destruction, or surgery. Proinflammatory cytokines, such as interleukin-1 beta, tumor necrosis factor-alpha, and interleukin-6, as well as prostaglandins and bloodborne molecules, such as lipopolysaccharide, communicate with the brain via either direct autonomic neural pathways, active transport of cytokines across the blood-brain barrier, second messenger systems in the blood-brain barrier, or via disruption of the blood-brain barrier.59-61 Recognition of these peripheral inflammatory stimuli initiates a cascade resulting in astrocyte, microglial, and endothelial activation, leading to production of additional inflammatory cytokines, reactive oxygen species, and expansion of the microglia population, culminating in neuroinflammation and ultimately neuronal damage.59,61 Advanced age, underlying dementia, and states of chronic inflammation may “prime” microglial cells, resulting in an exaggerated inflammatory response.59-61 In addition, systemic inflammation results in endothelial damage leading to thrombin formation and vasoconstriction with resultant microvascular compromise.62 The combination of neuroinflammation and disruption of normal CNS perfusion may then impair neurotransmitter synthesis and release (particularly acetylcholine),50 impair oxidative metabolism, and deplete neuronal energy stores.52

Full access? Get Clinical Tree