KEY POINTS
Potentially the most important diagnosis with highest life-saving capability in medicine.
Challenging diagnosis requiring high clinical suspicion and quick, efficient use of diagnostic modalities.
Clinically, the typical pain, incongruous poor tissue perfusion despite hypertension, and/or evidence of aortic branch occlusion suggest the diagnosis.
Emergent control/support of blood pressure and pain is imperative.
Investigation with urgent CT angiogram or TEE to confirm diagnosis and complications.
Categorize as type A (ascending aorta involved) versus type B (only descending aorta involved) to direct definitive treatment.
Type A requires emergency cardiac surgical repair.
Type B managed with emergency medical management versus endostenting or surgery if complicated.
Long-term strict control of hypertension and surveillance important to identify need for late intervention and maximize long-term survival.
INTRODUCTION
Aortic dissection occurs much more frequently than previously appreciated and is actually the most common catastrophe affecting the aorta, occurring 2 to 3 times more commonly than acute abdominal aortic aneurysm rupture.1-3 Although the diagnosis is sometimes obvious, the majority of cases are not clear-cut and the patient’s survival will depend on a high index of suspicion by the physician despite a myriad of different clinical presentations. Time is of the essence as the mortality is 50% for the first 48 hours without treatment and 85% to 90% over 3 months. The typically hypertensive patient must have their blood pressure and pain controlled quickly followed by rapid diagnosis with definitive imaging and immediate relegation to the appropriate therapy of either emergency surgery or medical management/endostenting.
PATHOGENESIS
Previously, aortic dissections were referred to as dissecting aneurysms, as originally coined by Laënnec. This is a misnomer in that the pathology is a dissecting hematoma that separates the intima and inner layers of the media from the outer medial and adventitial layers (Fig. 42-1). The intima is therefore not aneurysmal, and is, if anything, narrowed. Blood invades the media through a tear in the intima and proceeds ante- or retrogradely through the aortic wall, forming a false lumen.4 In type A dissections (originating in the ascending aorta) the hematoma commonly spirals around the right and posterior aspects of the ascending aorta, supraposteriorly along the arch, and then down the left and posterior aspects of the descending aorta. The hematoma may then have several serious sequelae. It may rupture into the pericardial space causing tamponade or into the pleural space with exsanguinating hemorrhage, especially in type B dissections (begin after the left subclavian artery). This occurs less frequently than expected because the adventitial layer represents 66% of the overall strength of the aortic wall. It may also cause occlusion of aortic branch arteries or prolapse of one or more of the aortic valve cusps, resulting in acute aortic insufficiency.
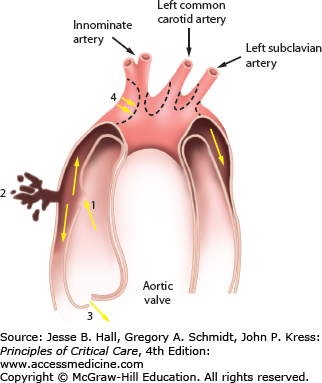
FIGURE 42-1
Aortic dissection begins with an intimal tear (1) leading to a hematoma that separates the layers of the aortic wall. The sequelae are rupture through the adventitia into the pericardium (2), prolapse of the aortic valve cusps leading to aortic insufficiency (3), compression of the aortic branch vessels (4), and aneurysmal dilation of the ascending arch and descending aorta.
Generally the tear is due to either a weakening of the wall of the aorta, an increase in luminal shear stress, or both. Weakening of the aortic wall can occur as the result of medial degeneration or iatrogenic injury. Medial degeneration (cystic medial degeneration or necrosis) is manifested by the loss of smooth muscle cells and accumulation of basophilic amorphous material with or without associated “cysts” in the aortic media. This is believed to be due to inborn errors of metabolism (Marfan or Ehlers-Danlos syndrome). There is a reduction in the cohesiveness of the layers of the aortic wall as a result. Other causes of reduced wall strength causing aortic dissection include annuloaortic ectasia, coarctation, and pregnancy (especially in the third trimester). A bicuspid aortic valve is present in up to 22% of type A dissections and dissections occur 5 to 10 times more commonly than in trileaflet aortic valves.5 The aortic wall has been found to be abnormal in these patients with increased expression of genes associated with cell death (eg, the gene for interleukin-1B) causing reduced collagen content similar to that seen in Marfan syndrome.6 Iatrogenic injuries occur during open heart surgical procedures at any point where the aorta is invaded, such as the aortotomy for an aortic valve replacement or the proximal anastomosis of an aortocoronary bypass graft. Stresses applied to the aortic wall increase wall tension and lead to dissections. Most important are intraluminal shear stresses, which are related both to the level of the systolic blood pressure and to the steepness of the aortic pulse wave.2 This is referred to as dP/dTmax and represents the speed with which the maximal systolic pressure is attained in the aortic root. As this increases, so too does the shear stress on the ascending aorta.7
Human genetic and biomarkers studies and findings from animal models suggest the possibility of identifying some genetic and biomarker risk factors for dissection. This could potentially lead to improved targets for drug development to stabilize the aortic wall in high-risk patients and prevent dissections.8
CLASSIFICATION
Dissections are classified by timing and location to identify the morbidity and mortality for the specific lesions.
Acute: <2 weeks
Chronic: >2 weeks
Acute dissections are very high-risk lesions with an estimated mortality for type A of 50% for the first 48 hours (∼1% per hour).
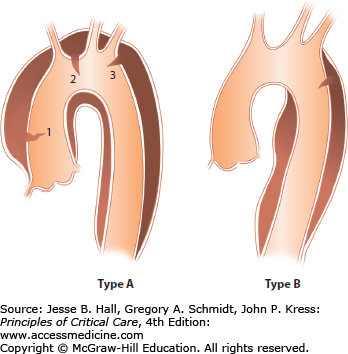
Type A (Fig. 42-2A-E): The ascending aorta is involved independent of the site of the intimal tear (since 15% of transverse arch and 5% of descending aortic tears will involve the ascending aorta by retrograde dissection), and may include the aortic arch and part or all of the descending thoracic and abdominal aorta. In autopsy series, type A dissections outnumber type B dissections almost 2:1.9
Type B (Fig. 42-3A and B): Descending aorta (beyond the left subclavian artery).
This classification system, proposed by Daily and colleagues and popularized by the Stanford group, replaces the original system proposed by DeBakey (Fig. 42-4). The classification system is based on the risk of sudden death from the dissection, which is highest in type A. Here the dissection may cause tamponade or severe aortic insufficiency with congestive heart failure as well as coronary thrombosis, especially involving the right coronary artery, with acute myocardial infarction. Type B dissections do not have these risks and generally can be approached and managed conservatively. As a result, therapeutic interventions are dependent on location with almost all type A dissections requiring urgent operative intervention, whereas type B dissections are managed primarily pharmacologically or with endostenting and, less commonly, surgery for specific complications. Long-term surveillance is imperative to follow potential dilatation of the descending aorta (especially if the false lumen is patent), as in up to 40% of patients the type B dissections can lead to late death.10 Endostenting has been proposed as an intervention to improve survival in these patients.11-13
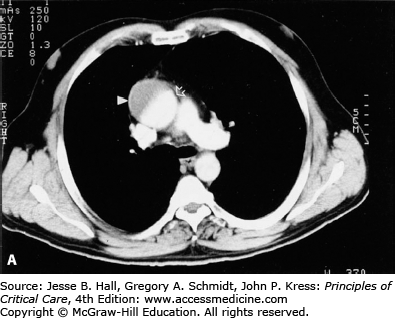
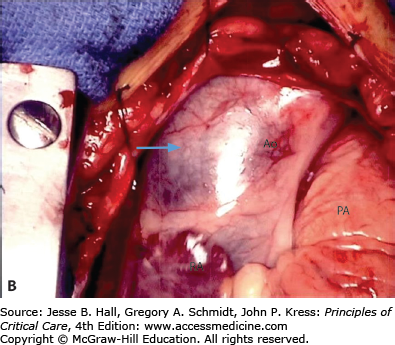
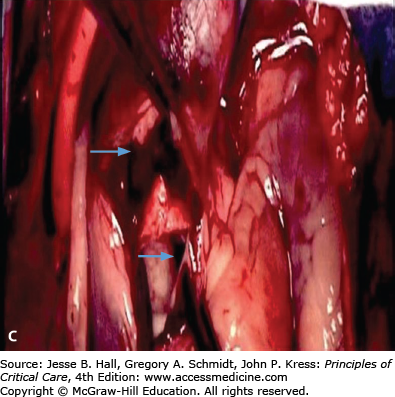
FIGURE 42-2
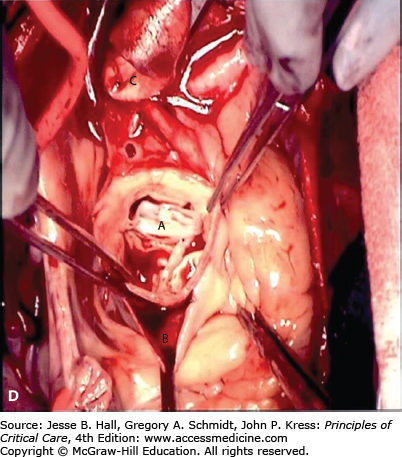
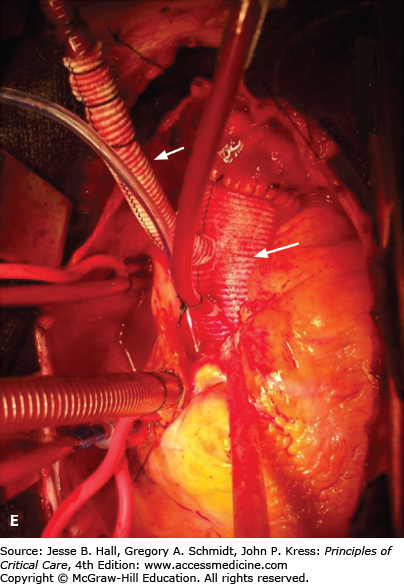
A. Contrast-enhanced CT scan of the thorax in a 66-year-old man with acute chest pain radiating to the back and left flank. A dilated aortic root and intimal tear are present. Contrast material fills the true lumen first (open arrow), while false-lumen filling is delayed (solid arrow). B. Type A dissection with blood (bluish discoloration—arrow) in the subadventitial layer. Ao, aorta; Pa, pulmonary artery; RA, right atrium. C. Aorta opened showing clot in false lumen (wide arrow) and true lumen (narrow arrow). D. Proximal aorta (ascending aorta has been resected) showing aortic valve leaflets (A), false lumen (B), and distal aorta (C). E. Interposed Dacron graft (large arrow) with sidearm (small arrow).
FIGURE 42-3
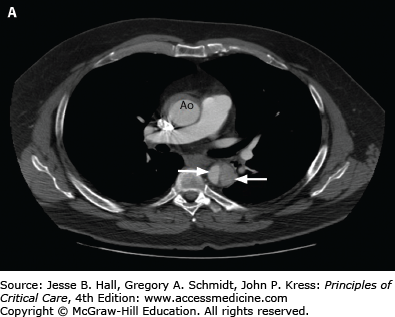
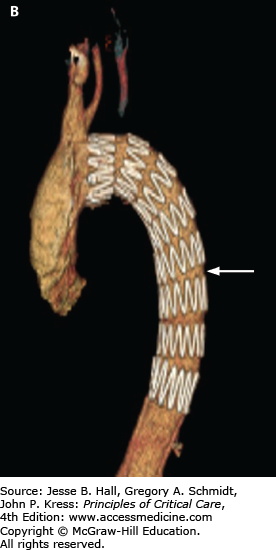
A. CT angiogram of chest in a 51-year-old man with severe back pain, hypertension, and reduced pulses in the femoral arteries showing type B dissection in descending thoracic aorta with false lumen (wide arrow) compressing the true lumen (narrow arrow) and normal ascending aorta (Ao). B. Endostent placed in the descending aorta (arrow) beginning just beyond the left subclavian artery. (Used permission of Dr. Benjamin Starnes, Chief, Division of Vascular Surgery, University of Washington.)
FIGURE 42-4
Classification of aortic dissection based on the presence or absence of ascending aortic involvement. Type A dissections involve the ascending aorta, and type B dissections do not. The intimal tear in type A dissections may be in the ascending aorta (1), the arch (2), or the descending aorta (3). Type A includes DeBakey types I and II. In type B dissection, the intimal tear is distal to the left subclavian artery origin. Type B dissections correspond to DeBakey type III. (Reproduced with permission from McGoon C. Cardiac Surgery. 2nd ed. Philadelphia, PA: FA Davis; 1987.)
AORTIC INTRAMURAL HEMATOMA
Aortic intramural hematoma (IMH) results from hemorrhage within the aortic wall without disruption of the intima. It is likely due to rupture of the vasa vasorum and may progress to rupture through the intima to form a classic dissection in up to 33% to 47% of cases while only 10% regress.14
It is an entity that is frequently confused with aortic dissection. The diagnosis, management, and prognosis of IMH remain debatable.15-17 Most authors believe the clinical course is similar enough to warrant treatment of IMH the same as a classical dissection (Fig. 42-5).18-21 The diameter of the aorta is important in that if the aortic diameter is <45 mm at 1 month follow-up, then most will resolve. Complications appear to occur mainly in patients with an aortic diameter over 45 mm.19 The same consideration is given to atherosclerotic penetrating ulcers. It is suggested that these ulcers, IMH, and dissection might all be related and should be treated in similar fashion.22,23
CLINICAL PICTURE
Men, particularly African-Americans, are at two to three times the risk of developing an aortic dissection as women. More than 90% will have a history of hypertension requiring treatment. The presentation of an acute dissection can be subtle, demanding great attention to detail to make the diagnosis, or classic and obvious. A new murmur of aortic insufficiency is present in 50% to 66% of type A dissections24 due to the loss of support of the valve at the commissures as the inner layer of the aorta collapses inward. A continuous murmur suggests rupture of the dissection into the right ventricle or atrium. The signs and symptoms are related to the location of the tear and the extent of the hematoma dissection. These are manifested mainly by pain, poor peripheral perfusion despite an increased blood pressure, and signs and symptoms of aortic branch occlusion.
Typically, the pain is either retrosternal or central interscapular back pain, but it may be epigastric. Classically, it begins in the chest, moves to the back, and then moves down to the abdomen or lower extremities as the dissection progresses, but this pattern is rarely seen. Patients describe the pain as “sharp,” “tearing,” or “knife-like,” and it is most often excruciating in intensity. To differentiate it from angina, the pain is maximal immediately upon onset, and it is often difficult to obtain relief with opiates. Clinical diagnostic accuracy may approach 90% if three basic questions are asked regarding the pain’s quality (tearing or ripping), radiation (beginning between the scapulae and radiating down the back), and the intensity at onset (abrupt onset of 10/10 pain).25
Patients frequently present with evidence of shock, with a cool, clammy periphery, ashen coloring, and depressed level of consciousness, and yet markedly elevated systolic blood pressure frequently exceeding 200 mm Hg. Most often this is due to reflex sympathetic discharge from the intense pain. It can occur, however, with myocardial infarction due to coronary artery occlusion (especially the right coronary artery) by the dissection, or from severe aortic insufficiency with congestive heart failure, which is present in 30% to 60% of patients with type A dissections. If the blood pressure is depressed, the dissection may have ruptured into the pericardium with tamponade (as occurs in up to 30% of type A dissections) or into the pleural space (left more often than right) with resulting hypovolemia. Hypotension occurred in >25% of patients with acute aortic dissection among patients enrolled in the International Registry of Acute Aortic Dissection (IRAD) and was associated with much higher rate of in-hospital adverse events.26 Cardiac tamponade is a life-threatening complication and the leading cause of death. Emergency echo-directed percutaneous drainage of pericardial effusions causing tamponade may be performed for hemodynamic instability but should not delay surgery.27,28
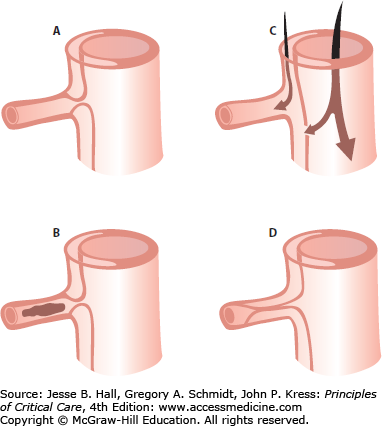
Approximately one-third of all patients will present with compromised flow to a major branch of the aorta as part of their presentation.29 The vessel may be sheared off or compressed, resulting in occlusion and/or thrombosis, or be perfused through the false lumen (Fig. 42-6).
FIGURE 42-6
Aortic branch occlusion mechanisms. A. Compression of the true lumen by the false lumen with a patent true lumen. B. Complete occlusion of the true lumen by the false lumen with thrombosis. C. Complete avulsion of the intima from the origin of the branch vessel with blood flow provided both from the false lumen and the true lumen via distal reentry. D. Complete occlusion of the true lumen by the false lumen beyond the branch orifice. (Reproduced with permission from Cambria RP, Brewster DC, Gertler J, et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg. February 1988;7(2):199-209.)
Table 42-1 lists the vessels affected and the manifestations. The dissection usually travels in a spiral motion down the thoracic aorta such that the celiac axis, superior mesenteric artery, and right renal artery remain intact. In type A dissections the innominate artery (and thus blood flow to the right carotid artery to the brain, causing cerebrovascular insufficiency, and the right subclavian artery to the right upper extremity, causing a pulse deficit) are the most frequently affected. A measured brachial pressure differential of greater than 20 mm Hg should lead the clinician to strongly consider the diagnosis of type A dissection. The dissection usually stops at the level of the iliac arteries, with the left iliac more often affected, leading to compromised blood flow to the left leg. Rarely is there only one tear in the aorta, and more commonly, the dissection has multiple reentry sites along its path down the aorta. The common femoral arteries are seldom dissected, an important issue at surgery, since cannulation of the femoral artery is one option for placing the patient on cardiopulmonary bypass (CPB).30 Fortunately, the visceral and renal vessels are affected in less than 3% of patients, since their involvement denotes a much increased mortality rate of 41% versus 27%.31 Neurologic sequelae are of particular concern. Some neurologic dysfunction, such as depressed level of consciousness or dizziness, is said to occur in 30% to 50% of patients.3 However, concrete focal neurologic deficits occur much less frequently (<10% overall), and may affect the central nervous system (CNS), spinal cord, or peripheral nerves. CNS deficits range from minor transient ischemic attacks to deep coma. Cerebrovascular accidents (CVAs) causing hemiparesis affect 5.5% to 6.7% of patients with type A dissections. They are primarily due to innominate-carotid artery occlusion, with the right side affected in two-thirds of cases. They can also be caused by emboli or low flow with thrombosis due to previous carotid stenosis. Paraparesis and paraplegia fortunately are rare (2% of type A), because they portend a very poor prognosis. Occasionally patients may present with vascular compromise foremost in their complaints and findings. A patient suffering an acute occlusion of blood flow into their lower extremity, particularly the left, with no definite clot or embolus found at surgery should be strongly suspected of having a dissection and investigated immediately. Unfortunately the physical findings classically associated with dissection are present in less than half of all cases, thereby necessitating a high index of suspicion to save these patients.32
INVESTIGATIONS AND DIAGNOSIS
Laboratory data are usually within normal limits in patients with acute dissection. The white blood cell count may be slightly elevated to 12,000 to 20,000/μL, most likely as a stress response. Electrocardiogram (ECG) interpretation may show left ventricular hypertrophy due to chronic hypertension, but other changes are rare. Acute ischemic changes should raise the concern of coronary artery involvement by the dissection in the patient with a typical history. Conversely, to avoid the dire consequences of misdiagnosis, any patient presenting to the emergency department with ECG changes suggesting myocardial ischemia (especially with evidence of right coronary artery involvement) should have their history considered carefully before immediately moving to urgent cardiac catheterization to treat the more prevalent condition of atherosclerotic coronary artery disease (CAD).
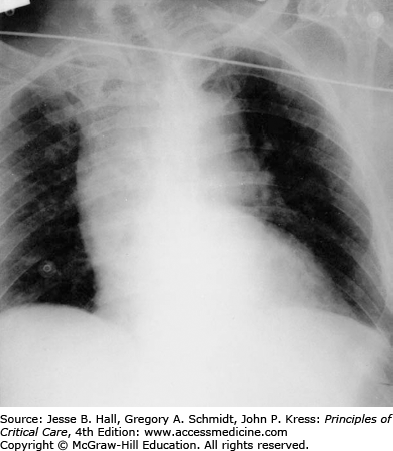
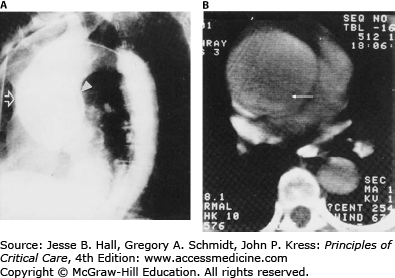
Imaging is a critical step in diagnosis, classification, and management of aortic dissection. Standard anteroposterior and lateral chest x-rays (CXR) often reveal a widened mediastinum (Fig. 42-7), although this may be absent in up to 40% of type A dissections. Classically, the aorta bulges to the right with type A and to the left with type B dissections. Occasionally a double rim of calcification may be present in the distal aortic arch or a pleural effusion may be present, left more commonly than right. This may be the result of a periaortic inflammatory reaction at the site of dissection, although frank blood (hemothorax) may be seen in cases of aortic rupture. Although the CXR may raise the suspicion for aortic dissection or support the clinical impression, it is rarely diagnostic. Consequently, a normal CXR on presentation should not delay solicitation of advanced imaging for exclusion of aortic dissection in the appropriate clinical setting.
Several imaging techniques including aortography, computed tomographic (CT) scanning, magnetic resonance imaging (MRI), and echocardiography are highly accurate for the diagnosis and classification of dissections.33 In the past, aortic angiography was commonly used as the initial definitive diagnostic method with high sensitivity and specificity (Fig. 42-8). However, it is now rarely used due to development of advanced non-invasive techniques with the added benefit of improved detection of the aortic IMH and penetrating ulcer variants.33-37
FIGURE 42-8
A. Aortogram (lateral projection). Grossly dilated ascending aorta (open arrow) with visible intimal flap (solid arrow). Note normal descending aorta. B. Contrast-enhanced CT scan of the thorax of the same patient. Arrow identifies intimal flap. (Reproduced with permission from Kotler N, Steiner RM. Cardiac Imaging: New Technologies and Clinical Applications. Philadelphia, PA: FA Davis; 1986.)
Since type A dissections are life-threatening surgical emergencies, it is critical to assess involvement of the ascending aorta.
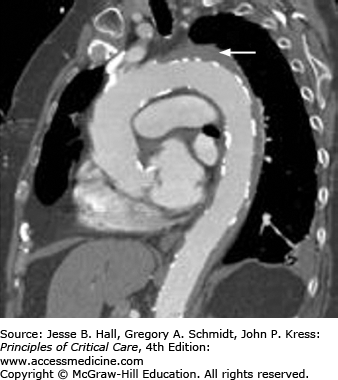
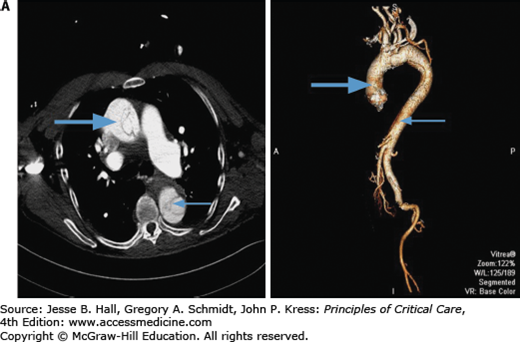
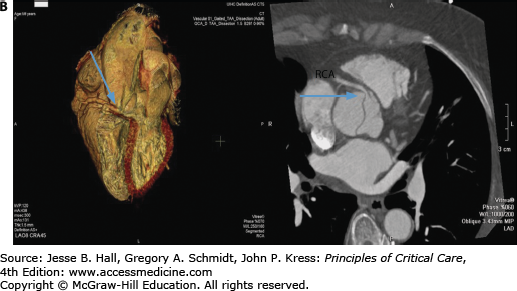
Contrast-enhanced CT scanning is readily available at most institutions and is the most commonly used modality to evaluate aortic dissections, particularly in patients with type B.38 Specific identification of true and false lumens with a flap is possible, as well as detection of pericardial effusion and accurate depiction of the extent of the dissection. The newer spiral (helical) CT scans that are now available in most hospitals yield excellent two- and three-dimensional images and should be electrocardiographically gated to reduce motion artifacts from a pulsating aorta (Fig. 42-9A and B).39 CT scanning is very accurate for the diagnosis of dissection (∼98-100% accuracy)32,33,40 and is superior to transesophageal echocardiography (TEE) for the detection of aortic branch involvement (∼96% accuracy).33 Unlike TEE, however, CT does not yield information regarding aortic insufficiency or left ventricular function and requires exposure to potentially nephrotoxic radiographic contrast and radiation and may be problematic in unstable patients.
Transthoracic echocardiography (TTE) has low sensitivity to diagnose aortic dissections due to limited visualization of the distal ascending aorta, aortic arch, and descending aorta. A dissection flap can be occasionally seen in the ascending aorta that yields the diagnosis (Fig. 42-10).36,41 Application of harmonic imaging and administration of contrast may improve the accuracy of TTE in diagnosis of ascending aortic dissection.42,43

Full access? Get Clinical Tree