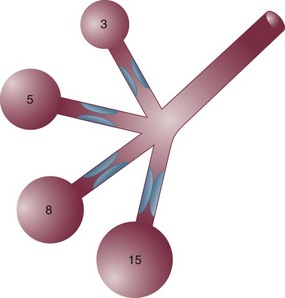
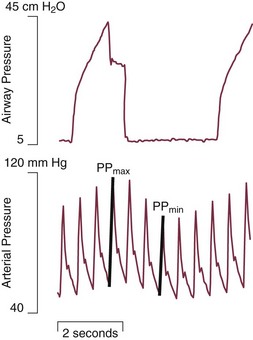
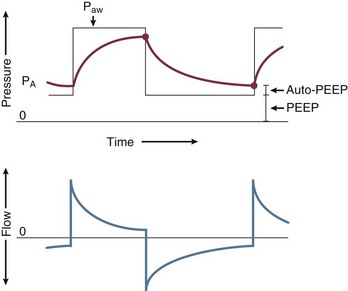
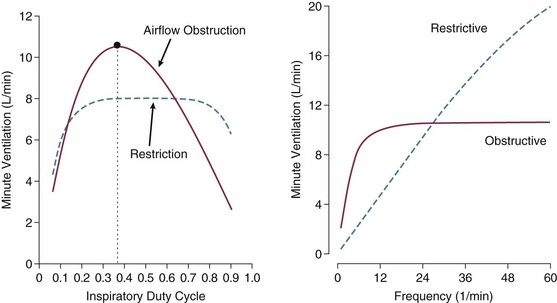
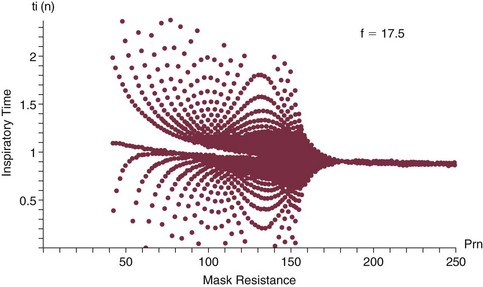
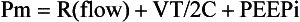10 SPECIAL CHALLENGES OF PATIENTS WITH SEVERE AIRFLOW OBSTRUCTION PROBLEMS AND HAZARDS OF VENTILATION WITH POSITIVE PRESSURE PRINCIPLES OF MANAGING THE VENTILATED PATIENT WITH SEVERE AIRFLOW OBSTRUCTION PRACTICAL MANAGEMENT OF THE VENTILATED PATIENT In this equation R = resistance; VT is tidal volume; C is respiratory system compliance (the inverse of elastance); and PEEPi is auto-PEEP, the positive end-expiratory alveolar pressure in excess of set PEEP because of dynamic hyperinflation (Fig. 10.1). Increased resistance to airflow is responsible (directly or indirectly) for many of the physiologic disturbances that typify this disease. Flow resistance, an important determinant of Pm, is increased by structural and functional narrowing of the airway. Structural changes include a reduced number of airway channels, as well as narrowed cross-sectional airway caliber. In this already narrowed tapering network of tubes, the additional reduction of airway caliber caused by mucosal edema, functional compression, increased bronchomotor tone, or secretion accumulation noticeably increases the work of breathing because resistance relates linearly to the airway length but inversely to the fourth power of airway radius. For similar reasons, resistance within these critically narrowed airways is highly volume dependent, so that loss of lung volume is accompanied by loss of elastic recoil tension, reduction of cross-sectional area, tendency for expiratory airway collapse, and major increases in the frictional workload.1,2 Expiration is normally a passive process that uses elastic energy stored during inflation to drive expiratory airflow. If the energy potential stored during inflation is insufficient to return the system to a relaxed equilibrium before the next inspiration begins, flow continues throughout expiration and alveolar pressure remains positive at end expiration, exceeding the clinician-selected PEEP value (Fig. 10.2).3–9 This positive distending pressure within the alveoli increases the driving pressure for expiratory airflow and increases lung volume, thereby helping to overcome airflow resistance. Unfortunately, such hyperinflation also places the expiratory musculature at a mechanical disadvantage. Furthermore, because the hyperinflating end-expiratory alveolar pressure encourages deflation, it must first be counterbalanced by positive pressure applied to the central airway or by negative pleural pressure before inspiration can begin.10,11 Thus PEEPi adds to the other components of the equation of motion to elevate the mean inflation pressure and inspiratory work of breathing. The process of air trapping contributes to an increase in the respiratory work of breathing in at least two other ways. Hyperinflation drives the respiratory system upward toward the least compliant portion of the pressure-volume relationship, incurring increased elastic work per liter of ventilation (see Fig. 10.1). At these higher volumes the lung approaches its elastic limit as the recoil tension of the distended rib cage becomes expiratory rather than inspiratory in nature. Finally, hyperinflation tends to convert more of the well-perfused (“zone 3”) lung into less-well-perfused tissue, thereby increasing ventilatory deadspace and the minute ventilation requirement. Generally, the resistance increase in patients with chronic AO and many of those with severe asthma concentrates within small airways.1 Yet for certain asthmatic patients, the central airways and larynx contribute impressively to total resistance, accounting for the helpfulness of helium-oxygen (heliox) mixtures in some (but not all) patients during exacerbated asthma.12 According to some reports, heliox helps to reduce air trapping in patients with COPD as well. Although there is some concern regarding the generalizability and accuracy of such observations, several mechanisms can be invoked, even if the primary site of expiratory obstruction is too peripheral for helium to reduce resistance there. These mechanisms include reduced inspiratory turbulence, faster expiratory flow in non–flow-limited channels with increased wave speed, modestly decreased CO2 production, and perhaps reduced associated gas trapping. When dynamic collapse occurs during tidal respiration and breathing requirements are high, there is little alternative to hyperinflation, CO2 retention, or both. At the chosen level of minute ventilation, maintaining the lower lung volume may be either too energy costly or physically impossible. For this reason, many patients with severe obstruction do not or cannot decrease their lung volumes when recumbent. Such considerations may help to explain the dyspnea experienced by most patients with severe AO on assuming horizontal positions. For the same recumbent angle, the lateral position allows slightly more decompression than does the supine position because lung volume influences airway caliber, airway resistance, and tendency for collapse as the lung deflates.13 Patients with obesity have a lower resting lung volume and therefore exhibit higher airway resistance and tendency for dependent atelectasis and symptoms when airways are narrowed by bronchoconstriction or disease. Likewise, patients with acute respiratory distress syndrome have higher than normal airway resistance in dependent zones. In fact, the distribution of gas trapping varies regionally throughout any diseased lung, depending on the local mechanical properties of the airways. Therefore, at the end of the expiratory cycle some zones remain patent, and some have sealed much earlier in the deflation cycle (see Fig. 10.2). Consequently, the end-expiratory value of auto-PEEP detected at the airway opening may not reflect the magnitude of gas trapping.14 Clues to the presence of regional closure are often seen when the airway is occluded at end expiration; the auto-PEEP value shows an atypically slow rise to its final value as quasi-occluded small airways decompress into the common airway. In such cases, PEEP often eliminates this characteristic. During volume-controlled ventilation, plateau pressure tracks hyperinflation more reliably than direct measurements of PEEPi. In some patients, especially those who passively receive ventilatory support, the problems presented by air trapping and dynamic hyperinflation are as much cardiovascular as pulmonary in nature. A relatively high fraction of the resulting positive alveolar pressure is transmitted to the pleural space, where it impedes venous return and confuses interpretation of hemodynamic pressure measurements made with pulmonary artery catheters (Fig. 10.3). Lung distention also adds to pulmonary vascular resistance, exacerbating the tendencies of patients with cor pulmonale toward low cardiac output and hypotension. Marked respiratory variation of systolic and pulse pressures during passive inflation indicates phasically adverse cardiac loading and strongly implies the possibility of dynamic hyperinflation (Fig. 10.4). Ventilation-perfusion ( In patients with exacerbated COPD or decompensated asthma, the oxygen consumed in the ventilatory task is disproportionate to the amount of mechanical work actually performed. The muscles of the hyperinflated ventilatory system are inefficiently aligned, force-length relationships of the shortened end-inspiratory fibers are suboptimal, and normally efficient coordination among the various muscles of the ventilatory group is often disrupted.14–16 The energy cost of breathing, therefore, is greatly increased for the pressure and mechanical work actually generated by the breathing effort. The hemodynamic sensitivity to positive-pressure ventilation of patients with AO arises for several reasons. The overexpanded lungs press outward on the chest wall, raising intrapleural pressure. When breathing efforts are silenced, as they are immediately after sedation and intubation, mean intrathoracic pressure abruptly changes from modestly negative to markedly positive. Increased pleural pressure raises the right atrial back-pressure to venous return. Simultaneously, increased peripheral vascular capacitance (caused in part by drug effects) and reduced peripheral vascular tone limit the rise in mean systemic pressure, the upstream driver of venous return. Blood pressure routinely falls, and cardiac output falls disproportionately to oxygen demand. Absolute values of measured central vascular pressures (central venous and wedge pressures) may therefore be misleadingly high and do not reflect intravascular filling and preload adequacy.7 Marked respiratory variation of systolic and pulse pressures with the ventilatory cycle (“paradox”) is a hemodynamic marker of relative hypovolemia caused by such mechanisms (see Fig. 10.4). Depending on choice of tidal volume, backup frequency, and set (and auto) PEEP, the afterload to right ventricular ejection may rise with any further lung expansion, whereas the tendency for alveolar deadspace creation is accentuated. Consequently, great care must be taken not to ventilate excessively and to provide adequate intravenous fluid support during this period. This advice pertains especially to patients with AO who require cardiac resuscitation.17 Pressure-targeted modes of ventilation, exemplified by pressure control, airway pressure release ventilation (APRV), and pressure support, have become increasingly popular to employ in the care of intubated patients, as well as in those receiving noninvasive ventilation by facemask. Because the development of auto-PEEP reduces the pressure difference between airway opening and alveolus that drives inspiration, it has a powerful influence on ventilation efficacy (Fig. 10.5). As already described, auto-PEEP varies not only with airway mechanics but also with the pattern of breathing and minute ventilation. For a fixed value of applied airway pressure, inspiratory tidal volume in patients with AO will be more sensitive to the frequency and the inspiratory time fraction (an expression of the inspiratory-to-expiratory [I : E] ratio) than are normal subjects or those with restrictive disease18 (Fig. 10.6). Faster breathing frequencies, for example, allow auto-PEEP to build, and this auto-PEEP must first be counterbalanced for inspiratory airflow to begin. If the patient is passive or the amount of inspiratory muscle force remains constant, delivered tidal volume falls as the auto-PEEP builds. This auto-PEEP/driving pressure interaction may result in an intriguing phenomenon resembling chaotic respiration during noninvasive ventilation with a leaky mask interface.19 The coupled PEEPi and tidal volume form a “feed-forward” system in which a building auto-PEEP of one cycle adversely influences the tidal volume of the next one. But this smaller tidal volume also reduces the auto-PEEP that follows that restricted cycle, which in turn allows the subsequent breath—the third in the cycle—to have a larger effective driving pressure and tidal volume, and the cycling variation continues. This may account for some of the wide variability in breathing rhythm often observed in these patients.20 If the mask leak volume is a function of the I : E ratio, it can be shown mathematically and experimentally that fractal and chaotic tidal volume delivery may occur, even when the patient’s effort and mechanics remain unchanged (Fig. 10.7).19 The consequences for comfort and sleep efficiency are likely to be significant, but clinical data are lacking on these issues at this time. Most patients hospitalized with exacerbations of asthma or COPD can be managed effectively by regimens that incorporate aggressive secretion clearance techniques, antibiotics, corticosteroids, intensified bronchodilators, hydration, cardiovascular support, secretion lubricants (e.g., guaifenesin), and supplemental oxygen. Noninvasive ventilation often helps as a temporizing measure for those with disease of mild-moderate severity, especially when cough is adequate to clear airway secretions and the patient is fully alert and accepting of a full facemask.21–26 Only a minority of such patients treated in this way need translaryngeal intubation and institution of mechanical ventilatory support unless the problem is complicated by coexisting cardiovascular, infectious, or neuromuscular problems. When mechanical ventilation is required, however, the rationale underlying certain key management principles can easily be understood against a background of the physiologic derangements already described. These principles are as follows: (1) provide adequate support for muscle rest at adequate PaO2
Ventilatory Management of Obstructive Airway Disease
Special Challenges of Patients with Severe Airflow Obstruction
Increased Work of Breathing
Dynamic Hyperinflation (Air Trapping)
Increased Minute Ventilation Requirement
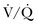 ) mismatching is widespread in patients with severe AO, reducing the efficiency of carbon dioxide elimination.1 It is not uncommon for the resting minute ventilation requirement to exceed 12 L per minute (twice the normal value) in patients with exacerbated asthma or extensive emphysema and strong chemical drives to breathe. Not only do such increases in ventilation requirement act as a linear cofactor in the work of the breathing equation already discussed, but the high minute ventilation requirement itself increases most components of inspiratory pressure: flow, elastance (the reciprocal of compliance), tidal volume, and auto-PEEP. It is not surprising, therefore, that enormous increases in the oxygen consumption rate of the ventilatory muscles have been observed in patients with obstructive lung disease. During exacerbations, the oxygen consumed by ventilation and the metabolic demands associated with heightened vigilance, agitation, or anxiety may double the total body oxygen consumption observed during fully supported breathing. The prevalent combination of impaired
) mismatching is widespread in patients with severe AO, reducing the efficiency of carbon dioxide elimination.1 It is not uncommon for the resting minute ventilation requirement to exceed 12 L per minute (twice the normal value) in patients with exacerbated asthma or extensive emphysema and strong chemical drives to breathe. Not only do such increases in ventilation requirement act as a linear cofactor in the work of the breathing equation already discussed, but the high minute ventilation requirement itself increases most components of inspiratory pressure: flow, elastance (the reciprocal of compliance), tidal volume, and auto-PEEP. It is not surprising, therefore, that enormous increases in the oxygen consumption rate of the ventilatory muscles have been observed in patients with obstructive lung disease. During exacerbations, the oxygen consumed by ventilation and the metabolic demands associated with heightened vigilance, agitation, or anxiety may double the total body oxygen consumption observed during fully supported breathing. The prevalent combination of impaired 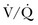 matching, hypoventilation, and diffusion impairment result in arterial oxygen desaturation that generally responds well to modest supplementation of inspired oxygen.
matching, hypoventilation, and diffusion impairment result in arterial oxygen desaturation that generally responds well to modest supplementation of inspired oxygen.
Reduced Mechanical Efficiency
Problems and Hazards of Ventilation with Positive Pressure
Interactions of Pressure-Targeted Modes with Auto-PEEP
Principles of Managing the Ventilated Patient with Severe Airflow Obstruction

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine