The prevalence of valvular heart disease is an increasingly burdensome problem and a common complicating comorbidity in the critically ill patient.
I. AORTIC STENOSIS (AS) refers to a narrowing of the valvular orifice, restricting flow from the left ventricle (LV) into the ascending aorta during systole. Etiologies of AS include senile degeneration (calcific), congenital bicuspid or unicuspid valves, and, rarely in North America, rheumatic disease.
A. Pathophysiology
1. A narrowing of the aortic valve orifice leads to restriction of flow from the LV into the ascending aorta. This obligates increased LV chamber pressures, resulting in increased LV wall tension, myocardial oxygen demand, and compensatory LV concentric hypertrophy with increased susceptibility to ischemia and arrhythmias.
2. Adequate coronary perfusion pressure becomes dependent on maintenance of diastolic pressure through preservation of systemic vascular resistance.
3. Increasing LV wall thickness subsequently leads to impairment of active relaxation and passive ventricular filling during diastole.
B. Diagnosis
1. Physical exam: Auscultation reveals a loud, late peaking systolic murmur best heard at the right upper sternal border, radiating to the carotids, with delayed carotid upstroke.
2. Symptoms: Patients present with dyspnea on exertion, angina, or presyncope/syncope. The onset of symptoms strongly correlates to disease progression and dramatic increase in risk of sudden cardiac death.
3. Diagnosis: Echocardiography is used to diagnose aortic stenosis but cardiac catheterization may also be used. Pressure gradients obtained via catheterization are typically lower than echo-derived gradients.
C. Severe Aortic Stenosis: Aortic valve area (AVA) less than 1.0 cm2, or 0.6 cm/m2 when normalized to BSA for extremes in body size. Mean gradients greater than 40 mmHg, or peak flow velocity greater than 4 m/s. Pressure gradients are flow dependent, and the severity may be underestimated in patients with decreased LV function.
D. Hemodynamic Management
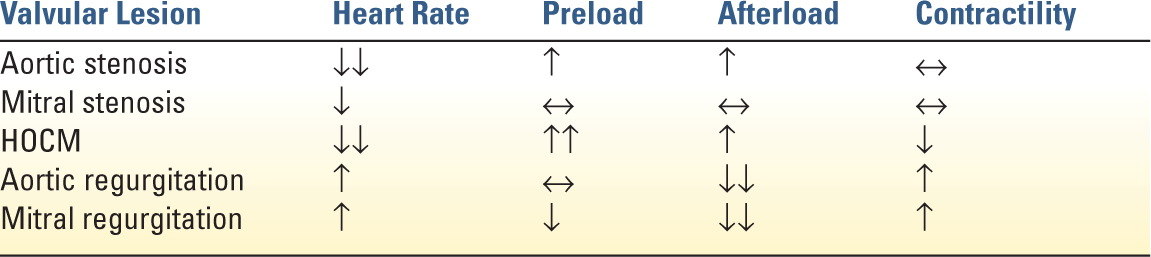
1. Heart Rate: Reduction is essential in patients with severe aortic stenosis. Tachycardia leads to significantly increased myocardial oxygen demand and decreased diastolic filling time. Extremes of bradycardia can be deleterious due to fixed stroke volume of the thickened ventricle or when it leads to decreased coronary perfusion.
2. Rhythm: Sinus rhythm becomes imperative as the hypertrophied LV becomes dependent on atrial contraction to maintain LV filling. In the setting of impaired LV relaxation, up to 40% of LV end diastolic volume is contributed by atrial contraction.
3. Afterload: Hypotension is poorly tolerated and should be treated immediately due to the high susceptibility of the hypertrophied myocardium to ischemia. First-line treatment is a pure vasoconstrictor such as phenylephrine to avoid tachycardia while improving coronary perfusion. In patients with decompensated heart failure and severe aortic stenosis, afterload reduction can be very carefully titrated with intensive hemodynamic monitoring to improve forward flow. In moderate or asymptomatic aortic stenosis, hypertension can be treated following standard medical guidelines.
4. Preload: Diastolic dysfunction from concentric hypertrophy may require elevated filling pressure for adequate stroke volume. However, in decompensate AS, aggressive fluid administration may lead to pulmonary edema.
5. Contractility: Inotropic support may be need in decompensated severe AS. However, inodilators such as dobutamine and milrinone can lead to dangerous decreases in systemic blood pressure and tachyarrhythmias.
E. Treatment
1. For mild to moderate asymptomatic aortic stenosis, medical therapy aimed at blood pressure and heart rate control following established guidelines is generally sufficient.
2. The definitive treatment for severe aortic stenosis is aortic valve replacement (AVR). Indications for surgical AVR or transcatheter aortic valve replacement (TAVR) include symptomatic severe AS or severe AS with heart failure. Surgical AVR is recommended for patients who are at low to intermediate surgical risk. TAVR may be considered in patients who meet indications for AVR but are at prohibitive surgical risk and have an expected survival of greater than 12 months.
3. Percutaneous balloon dilation: results in a modest reduction in the severity of AS, but is often complicated by severe AR, restenosis, and clinical deterioration. Percutaneous aortic balloon dilation as a bridge to AVR is controversial. Palliative balloon dilation is not recommended due to the associated morbidity and lack of improved mortality.
F. Postoperative AS Treatment Care: Although LV outflow resistance and LV cavity pressures should improve following AVR, LV hypertrophic remodeling may not resolve for months. Persistent LV hypertrophy requires higher filling pressures to maintain cardiac output, and the thickened myocardium remains susceptible to ischemia at low coronary perfusion pressures.
G. Hypertrophic Obstructive Cardiomyopathy (HOCM) is subvalvular dynamic obstruction of the left ventricular outflow tract (LVOT) caused by regional hypertrophy of the myocardium. Patients with HOCM are prone to lethal ventricular arrhythmias. Dynamic LVOT obstruction can lead to refractory hypotension and is exacerbated by ventricular underfilling, increased inotropy, decreased afterload, and tachycardia. Acute management includes increasing preload, increasing afterload with pure α-agonists such as phenylephrine, and administration of β-blockers, such as esmolol, to decrease inotropy and chronotropy.
II. AORTIC REGURGITATION
Aortic regurgitation (AR) is caused by incompetence of the aortic valve, resulting in retrograde blood flow from the ascending aorta into the LV during diastole.
A. Acute Aortic Regurgitation: Acute severe or moderate AR can lead to LV volume overload, decreased effective systemic output, and terminal ventricular arrhythmias. It is considered a surgical emergency. Acute AR is often a result of aortic dissection, infective endocarditis, trauma, or as a consequence of surgical or percutaneous intervention.
B. Chronic Aortic Regurgitation: Chronic AR is generally progressive. It may be the result of degenerative calcification, a bicuspid aortic valve, rheumatic fever, or aortic root dilatation. Chronic LV volume overload leads to LV remodeling. Initial eccentric LV hypertrophy will often progress to LV dilation in end-stage disease. Symptoms of severe disease include angina, congestive heart failure, and dyspnea on exertion.
C. Diagnosis
1. Physical exam: decrescendo diastolic murmur along left sternal border; widened pulse pressure; visible capillary pulsation with nail bed compression
2. Diagnosis and grading is made by echocardiography or catherization. Echocardiographic evidence of holo-diastolic flow reversal in the descending thoracic aorta by spectral Doppler, an AR vena contracta width of >0.6 cm, and an AR jet width/LVOT diameter ratio of >65% by color m-mode are consistent with severe AR by echocardiography.
D. Hemodynamic Management
1. Afterload: Reduction is critical and improves forward cardiac output when patient is acutely decompensated. In chronic AR, treatment of chronic hypertension is recommended; however, it does not alter the natural disease progression.
2. Heart rate: Elevation is beneficial to the management of decompensated AR. An elevated heart rate decreases regurgitant time, promotes forward flow, and may reduce diastolic volume overload. However, judicious β-blocker use may be indicated in the setting of aortic dissection to minimize propagation of the dissection.
3. Contractility: Inotropic support is often necessary to support heart failure in AR. Dobutamine and milrinone are often used for afterload reduction and for their positive chronotropic effect.
4. Preload: Fluid administration should be carefully regulated to prevent volume overload.
E. Treatment
1. Acute AR is often poorly tolerated and is considered a surgical emergency. Aortic valve replacement may be indicated for treatment of chronic severe AR when patients are symptomatic or have indications of LV systolic dysfunction or severe LV dilatation.
F. Anticoagulation after Aortic Valve Replacement
1. Mechanical valves—Long-term anticoagulation with low-dose aspirin 75 to 100 mg daily, plus warfarin with a targeted INR goal of 2.0 to 3.0 is recommended. If anticoagulation needs to be interrupted, the time with subtherapeutic INR should be minimized. Bridging with heparin is not generally required.
2. AVR with additional risk factors: hypercoagulable state, atrial fibrillation, prior thromboembolic event, or older-generation AVR—A higher INR goal of 2.5 to 3.5 is recommended. Use of heparin to bridge interruptions of anticoagulation is recommended.
3. Bioprosthetic AVR—Long-term, low-dose aspirin 75 to 100 mg daily, with warfarin—INR goal of 2.5 for the first 6 months after AVR.
4. Bioprosthetic TAVR—Clopidogrel 75 mg and low-dose aspirin 75 to 100 mg daily for the first 6 months.
III. MITRAL STENOSIS
Mitral stenosis (MS) results from obstruction of LV inflow through the mitral valve during diastole. Common etiologies of MS include rheumatic heart disease and senile calcific stenosis. MS commonly leads to elevated left atrial and pulmonary arterial pressures, which may lead to pulmonary edema and right ventricular (RV) dysfunction.
A. Diagnosis
1. Physical Exam: loud S1, opening snap after the S2, with a diastolic murmur best heard at the apex
2. MS may present with atrial fibrillation from left atrial distension, orthopnea, dyspnea on exertion, or heart failure. Hemoptysis is also a possible presentation from pulmonary hypertension. The appearance of symptoms is an indicator of significantly increased mortality in untreated disease.
3. Assessment with echocardiography or angiography is recommended. Exercise testing is also performed because transvalvular pressures are highly dependent on heart rate and hemodynamic loading. RV and LV performance and assessment for left atrial thrombus is also important. Severe MS is defined as a valve area <1.5 cm2 and very severe as <1.0 cm2.
B. Hemodynamic Management
1. Heart Rate: Lowering the heart rate facilitates diastolic filling of the LV. This improves forward flow and reduces pulmonary congestion. If pacing is needed, a longer PR time (0.2 seconds) may allow for better filling across the mitral valve.
2. Preload: Maintenance of adequate preload is critical for adequate filling through the stenotic mitral valve. Pulmonary artery occlusion pressures will be elevated but will not accurately reflect LV end diastolic filling pressure. However, the pulmonary vascular congestion resulting from the MS predisposes the patient to pulmonary edema so preload must be titrated carefully.
3. Contractility: If inotropic support for RV or LV failure is needed, use of drugs without increased chronotropy, such as digoxin or milrinone are advantageous.
4. Arrhythmia: Atrial fibrillation is common in MS and may be difficult to control. Unfortunately, ventricular filling may be highly dependent on atrial contraction in these patients. Rapid ventricular rates should be avoided and if rapid rates are associated with hemodynamic instability, the rates need to be treated aggressively.
C. Treatment
1. Percutaneous mitral balloon commissurotomy can be considered for symptomatic patients with severe MS and favorable anatomy. Mitral valve replacement or repair is considered for patients who have failed or are not candidates for percutaneous mitral balloon commissurotomy.
D. Anticoagulation with MS
1. Because of the high risk of embolic events, long-term anticoagulation is recommended in patient with MS and one of the following: atrial fibrillation, prior embolism, or left atrial thrombus. INR goal 2–3.
IV. MITRAL REGURGITATION
Mitral regurgitation (MR) is insufficiency of the mitral valve resulting in backflow during systolic ejection of the LV into the left atrium. This causes elevated pulmonary hypertension and ultimately right heart failure. The compensatory response of the LV results in dilation and eventually systolic dysfunction.
A. Acute Mitral Regurgitation is poorly tolerated and often presents with sudden hemodynamic decompensation. The sudden regurgitant flow of blood into the pulmonary circulation leads to pulmonary edema. The loss of forward flow leads to systemic shock. Acute MR can occur with papillary muscle or cordae tendinea rupture or dysfunction, often in the setting of an inferior myocardial infarction or valvular damage secondary to infective endocarditis. Early surgical repair is often indicated for severe acute MR.
B. Chronic Mitral Regurgitation: results in eccentric hypertrophy of the LV allowing it to accept the volume overload without major increases in LV end diastolic pressure. Chronic MR is characterized as primary if it is the result of dysfunction in the valvular apparatus, or secondary due to severe LV dilation and dysfunction. The etiology of disease has significance for definitive treatment. Because a fraction of the stroke volume is regurgitant, a normal ejection fraction (EF) is elevated in MR, 70%. When EF declines to 60% or end-systolic diameter is greater than 40 mm, LV dysfunction is inferred.
C. Diagnosis
1. Physical exam: Holo-systolic murmur is best heard at the apex with radiation to the left axilla. A hyperdynamic point of maximal impulse is often palpable. In severe acute MR, the murmur may be absent due to the rapid equilibration of pressure between the left atrium and LV.
2. Echocardiography is the primary method for diagnosis of acute MR, with transesophageal imaging indicated if transthoracic echocardiography is equivocal.
D. Hemodynamic Management
1. Afterload: reduction is essential for the management of MR. Reduced afterload promotes forward flow and reduces regurgitant fraction of the stroke volume. Nitroglycerin or calcium-channel blockers are often used in acute management. In severely unstable patients, intra-aortic balloon pumps (IABP) can be very effective by reducing effective afterload while preserving coronary perfusion.
2. Heart rate: higher heart rates decrease regurgitant time and reduce end diastolic volume preventing LV overdistension.
3. Preload: Volume status must be carefully balanced between adequate filling and overdistension of the LV that may dilate the MV annulus and worsen the MR.
4. Contractility: Inotrophic agents that also have chronotropic and afterload-reducing effects, such as milirinone or dobutamine, can be effective in improving forward flow in patients with heart failure from LV dysfunction.
5. Pulmonary hypertension: often occurs in severe MR and may lead to right heart failure. Avoidance of factors that elevated pulmonary artery pressures is important, such as hypoxia, hypercarbia, and acidosis. Use of pulmonary artery dilators such as inhaled nitric oxide or prostaglandin E1 may be considered.
E. Treatment
1. Mitral valve replacement or repair is indicated for patients with primary severe MR who are symptomatic or has signs of LV dysfunction. The treatment for secondary MR is less defined. It involves treatment of the underlying heart failure and possible cardiac resynchronization therapy.
F. Postoperative MVR Care
1. After repair or replacement of MR, the entire LV stroke volume ejected against the high systemic pressures results in higher effective afterload. This may unmask LV dysfunction and result in heart failure. Inotropic or IABP support may be required. Atrial fibrillation is poorly tolerated and antiarrhythmic or atrial overdrive pacing may be needed.

Full access? Get Clinical Tree