Fig. 66.1
Microvascular compression. A view of the right cerebellopontine angle including cranial nerves and vascular structures. Abnormal contact of a vessel (asterisk) with the trigeminal root entry zone, is thought to underlie trigeminal neuralgia. The most common offending artery is the superior cerebellar artery. SCA superior cerebellar artery, SPV superior petrosal vein, AICA anterior inferior cerebellar artery, PICA posterior inferior cerebellar artery
Two factors contribute to the increasing likelihood of vascular compression of a cranial nerve with age: the first is the increasingly dolichoectatic nature of cerebral arteries with age as the result of vascular disease; the second is the fact that the brain settles within the cranial vault in advanced age. The hypothesis that demyelination is crucial to the pathophysiology of the disease was generated by early observations that a disproportionate number of patients with multiple sclerosis suffered from trigeminal neuralgia [16]. Indeed, ultrastructural histological studies of trigeminal nerve root specimens in subjects with vascular compression demonstrate focal loss of myelin, oligodendrocytes, and astrocyte processes, with close proximity of axons to each other [17, 18]. These closely opposed axons without any substantial myelin sheath are prone to ephaptic coupling, which then leads to either stimulus-induced or constitutive activation [19, 20]. It is this aberrant firing of constituent peripheral neurons that is likely responsible for the stabbing paroxysms of facial pain. Furthermore, it is likely that the pulsatile compression of demyelinated axons by an artery is the instigating factor for these aberrant impulses.
While trigeminal neuralgia is the most common microvascular compressive disorder , there are a host of other cranial nerve compression syndromes thought to share a common pathophysiology. These include the following: hemifacial spasm (facial nerve), glossopharyngeal neuralgia (glossopharyngeal nerve), geniculate neuralgia (nervus intermedius), superior oblique myokymia (trochlear nerve), vestibular paroxysmia or hyperacussis or tinnitus (vestibulocochlear nerve), spontaneous gagging (vagus nerve), spasmodic torticollis (accessory nerve), and even neurogenic hypertension (lateral medulla) [21–28].
Trigeminal neuralgia is a clinic diagnosis; however, magnetic resonance imaging should be performed in all patients with neuralgic facial pain in order to exclude secondary causes such as aneurysm, vascular malformation, demyelinating disease, or various tumors, which can include vestibular schwannoma, meningioma, epidermoid cyst, etc. In many cases, the affected trigeminal nerve can be found to be thinned-out on high resolution T2-weighted MRI cisternography. Additionally, an offending dolichoectatic vessel is often found to be in contact with, or in close proximity to, the cisternal segment of the trigeminal nerve (Fig. 66.2). The diagnostic accuracy of MRI to identify a source of vascular contact with the fifth nerve has variable reports in the literature [29–35]. In one pooled analysis of seven studies, abnormal vascular compression was found in 131/170 cases, with an overall sensitivity of 77% and specificity of 71% [36]. Conventional computed tomography cisternography, with intrathecal contrast administration, may be helpful in patients unable to undergo MRI. The presence of a vessel in contact with the trigeminal nerve may aid in pre-surgical planning. However, the abscess of such a finding on imaging should not exclude a patient from neurosurgical exploration of the posterior fossa, as the offending vessel may often be a small and unnamed artery. Endoscopically assisted surgical exploration of the superior cerebellopontine cistern may aid in the identification of a neurovascular conflict [37, 38].
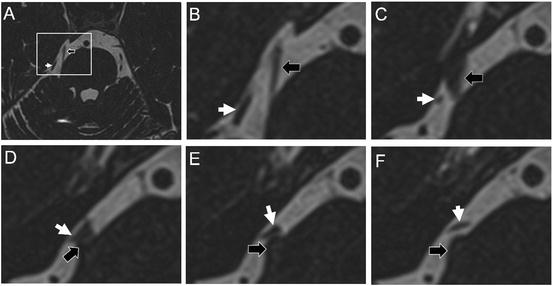

Fig. 66.2
High-resolution MRI. In many cases, the affected nerve can be seen to be thinned-out in the prepontine cistern. High resolution T2 weighted sequences often reveal loop of vessel in close proximity to the nerve. Absence of this finding, however, should not exclude patients from undergoing surgical exploration of the posterior fossa. In image (a) a high resolution T2 weighted image demonstrates the superior cerebellar artery (white arrow) in close contact with the trigeminal nerve (black arrow). Figs. (b–f) are sequential axial sections of the area demarcated by the inset in Fig. (a)
Medical Therapy
Early pharmacologic therapies for trigeminal neuralgia were severely limited. Fothergill proposed administering the bark of the cinchona tree, which contains quinine [6]. Other therapies specifically proposed for trigeminal neuralgia include ether, opium, arsenic, and conium maculatum [39]. Patients treated with stilbamidine for visceral leishmaniasis were known to experience bilateral trigeminal neuropathy, which led to the use of this agent as a treatment for trigeminal neuralgia despite significant side effects [40, 41]. Michel Bergouignan drew parallels between the paroxysmal nature of tic douloureux and epilepsy and began to treat the disease with phenytoin with some success [42]. The sodium channel blocker carbamazepine was shortly thereafter found to be even more effective [43].
Carbamazepine remains today the first-line treatment for trigeminal neuralgia. To date, four trials have prospectively compared treatment with carbamazepine versus placebo, altogether examining 147 subjects [44–47]. In these studies, 58–100% of participants achieved complete pain relief, with a number needed to treat (NNT) of two. The drug is typically initiated at 100–200 mg twice daily and is then escalated in increments of 200 mg daily until pain relief has been achieved, with the maximum recommended dose being 1200 mg daily. Typical adverse effects of carbamazepine include nausea and drowsiness; more concerning but rare side effects include agranulocytosis, aplastic anemia, and Stevens-Johnson syndrome. Oxcarbazepine is a derivative of carbamazepine which may be better tolerated. Two randomized controlled trials have demonstrated that both drugs are equally efficacious, with 88% of patients attaining a reduction of paroxysms by >50% [48, 49].
Second-line medical therapies are baclofen, lamotrigine, tizanidine, and pimozide—all of which have class I or class II evidence for therapeutic benefit in trigeminal neuralgia [50–53]. Open-label studies with class III or IV evidence suggest some benefit from multiple other antiepileptic drugs including phenytoin, valproic acid, gabapentin, clonazepam, pregabalin, and topiramate. Opioid medications and non-steroidal anti-inflammatory agents are generally not efficacious against neuropathic pain, but opioid pain medication may be helpful in patients with acute exacerbations as an adjuvant therapy to antiepileptic drugs. Intravenous administration of phenytoin or fosphenytoin, lidocaine, or carbamazepine may also be used for acute exacerbations.
Surgical Treatment
The paucity of early effective pharmacological therapies for neuropathic pain, coupled with the often debilitating nature of the disease, created an historical environment that invited creative surgical solutions for trigeminal neuralgia. In the mid-eighteenth century, George Maréchal, first surgeon in the court of Louis XIV, and his contemporary Nicolas André, both attempted to lesion the infraorbital nerve in patients with tic douloureux unsuccessfully. Shortly thereafter in 1768, Dussans and Veillard similarly transected the infraorbital nerve in two patients with facial pain, but without clinical effect [2]. In the early part of the next century, anatomists Charles Bell and François Magendie meticulously described the anatomy of the trigeminal nerve and thereby allowed for renewed efforts by surgeons. The first successful surgery for trigeminal neuralgia was performed by the American surgeon John Murray Carnochan in 1856 [54]. Carnochan hypothesized that the Gasserian ganglion was a “generator of nervous power of which, like a galvanic battery, it affords a continuous supply; while the branches of the ganglion under the influence of the diseased trunk, serve as conductors of nervous sensibility” [55]. Thus, he performed a trans-facial approach through the infraorbital foramen and maxillary sinus to extirpate the second division of the trigeminal nerve and the trigeminal ganglion. The first of Carnochan’s patients, who was himself a French physician, reported back over a year later that he remained pain-free [56].
By the end of the nineteenth century, the world’s prominent surgeons and indeed founders of neurosurgery had taken interest in the disease. Subsequently, they incrementally developed more refined approaches to the semilunar ganglion and the trigeminal nerve root. In 1890, William Rose published a report describing an infratemporal fossa approach to the foramen rotundum and foramen ovale [57]. Victor Horsley accessed the trigeminal ganglion for resection through a middle fossa craniotomy and intradural route [58], although this technique was prone to bleeding from the cavernous sinus. Frank Hartley and Fedor Krause contemporaneously with each other contributed to reducing the morbidity of the operation significantly by performing a subtemporal extradural exposure of Meckel’s cave for neurectomy and ganglionectomy [59, 60]. Harvey Cushing performed a similar extradural subtemporal procedure to Hartley and Krause, but approached the trigeminal ganglion partly through the infratemporal fossa, below the middle meningeal artery [61]. Finally, Charles Frazier at the University of Pennsylvania improved upon the technique in several ways. First, he performed a retrogasserian neurotomy of the trigeminal nerve rather than a complete ganglionectomy, allowing for preservation of the portion of the sensory root and ganglion supplying the first division and ophthalmic nerve as well as reducing the incidence of anesthesia dolorosa. Second, he was meticulous to preserve the portio minor, at times even using direct electrical stimulation to identify the motor root. The so-called Spiller-Frazier technique remained a favorite and durable treatment for trigeminal neuralgia in the following decades.
Walter Dandy, however, invented an entirely different way to access the trigeminal root through the posterior fossa, which he termed a “cerebellar approach.” Dandy performed a lateral sub-occipital craniectomy to enter the superior cerebellopontine cistern. There, he could visualize the cisternal segment of the trigeminal nerve from the root entry zone at the pons up to its entry into Meckel’s cave. Dandy’s original operation described partial sectioning of the portio major [62, 63]. His operation was a technical feat in this early era of neurological surgery before the advent of the operating microscope and microsurgical techniques. Indeed, the inability of most contemporary surgeons to successfully operate in the cerebellopontine angle limited the so-called cerebellar approach from replacing the Spiller-Frazier technique . Dandy’s unique vantage point in the posterior fossa, however, allowed him to discover vascular compression of the trigeminal nerve in 66 out of 215 cases [14]. This crucial observation would fall into relative obscurity for years to come.
Armed with the operating microscope, Peter Jannetta and colleagues described compression of the trigeminal nerve at its root entry zone at the pons by tortuous arteries in his series of neurotomies for trigeminal neuralgia [15] and prompted a re-examination of Dandy’s initial hypothesis. Additionally, the trigeminal root was often observed to be thinned-out at the site of compression, which correlated with some hypotheses that demyelination might play a key role in the pathophysiology of trigeminal neuralgia.
Based upon his observations, Jannetta conceived of a procedure to decompress the trigeminal nerve as an alternative to creating a destructive lesion. He approached the nerve through either a retrosigmoid craniotomy similar to Dandy’s operation or with a middle fossa transtentorial approach. In nearly every case, Jannetta claimed that an abnormal dolichoectatic vessel, or a small normal appearing vessel, contacted the fifth nerve at its root entry zone. He placed a cushion of polytetrafluoroethylene (PTFE) between the nerve and the offending artery, in order to decompress the nerve and to dampen pulsatile transmission (Fig. 66.3) [23, 64]. Over time, any skepticism within the neurosurgical community was overcome, and microvascular decompression (MVD) became overall the most effective and resilient procedure for trigeminal neuralgia. Subsequent neurosurgeons such as Takanori Fukushima further refined the technique and became effective at decompressing the trigeminal nerve through increasingly smaller craniotomies [65]. Its principle was extended to include other cranial nerve compression syndromes including hemifacial spasm, glossopharyngeal neuralgia, and more.
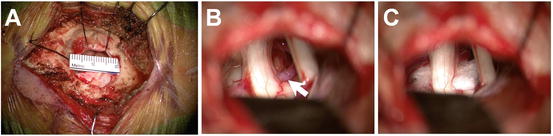

Fig. 66.3
Microvascular decompression. The cerebellopontine cistern can be accessed through a small retrosigmoid craniotomy (b). The fifth nerve (left), and seventh-eighth nerve complex (right) can be easily visualized. An ectatic loop of the superior cerebellar artery (arrow) can be seen making contact with the ventral aspect of the trigeminal root entry zone near the pons (b). A PTFE cushion is placed between the offending artery and the nerve in order to decompress the nerve and dampen pulsatile transmission (c)
Subsequent studies demonstrate that both short- and long-term outcomes from microvascular decompression for trigeminal neuralgia are excellent. The largest series studying this operation was a prospective trial published in 1996, which included 1885 patients [66]. In the vast majority of cases (75%), the aberrant artery compressing the fifth nerve was found to be a loop of the superior cerebellar artery (SCA) . Other possibilities included the anterior inferior cerebellar artery (AICA) (10%), the posterior inferior cerebellar artery (1%), the basilar artery (1%), and the labyrinthine artery (<1%). In up to 15% of cases, however, there was a small and unnamed artery at the root entry zone. Also, in 68% of cases, the petrosal veins were found to be compressing or abutting the nerve, although the significance of this is unclear given that these veins transmit no pulsatile force, which is thought to be a key component of the underlying pathophysiology.
In this large prospective study, over 98% of patients experienced pain relief in the immediate post-operative time frame, and there was complete freedom from any neuropathic pain in 82% of subjects. At 1 year post-operatively, 75% of patients remained completely pain-free, and an additional 9% had a good outcome with only occasional episodes of pain requiring no medication. At 10 years, 68% of patients persisted with an excellent or good outcome.
These data demonstrate the durability of microvascular decompression without necessitating any destructive lesion of neural tissue. The lack of immediate post-operative relief, female sex, venous compression without arterial compression, and long-standing pre-operative symptoms (>8 years) are all significant predictors of recurrence of tic douloureux after microvascular decompression. Prior radiofrequency ablation of the nerve or ganglion did not influence the primary outcome; however, patients with prior ablative procedures were more likely to experience persistent post-operative dysesthesias. Recurrence of symptoms after microvascular decompression is most commonly associated with recurrence of vascular compression, or more rarely with PTFE-induced granuloma [67, 68]. The majority of recurrences occur early on within the first 2 years.
With operator experience, microvascular decompression is a safe operation. In a meta-analysis of six studies prospectively examining the procedure, the mortality rate was 0.2% [36]. Other significant but rare complications include cerebellar hemorrhage or edema (0.6%), hearing loss from cranial nerve eight palsy (3.7%), facial weakness from cranial nerve seven palsy (0.6%), cerebrospinal fluid leak (1.7%), and venous sinus thrombosis (0.3%). Both intraoperative monitoring of auditory-evoked potentials [69–71] and minimizing cerebellar retraction [70, 72] may reduce the incidence of hearing loss. Minor complications include chemical aseptic meningitis (10.9%), which is easily treated with steroids, and decreased facial sensation (3.8%) [36]. There is virtually no incidence of corneal numbness or keratitis. As with any technically difficult operation, operator experience and volume of procedures performed at the institution may also have an important bearing on reducing perioperative morbidity [73]. The long-lasting durability of pain relief, relative paucity of facial numbness or facial dysesthesias, and the elimination of anesthesia dolorosa are all important advantages of microvascular decompression over destructive procedures [74, 75].
Percutaneous Rhizotomy
If early surgical techniques were focused on creating destructive lesions, such as neurotomies and ganglionectomies, then it would make sense to pursue less invasive means of creating lesions, ultimately leading to percutaneous rhizotomy by various methods. At the end of the nineteenth century, there were multiple reports of chemoneurolysis using chloroform, osmic acid, and alcohol [2]. Cutaneous injections of alcohol into the peripheral divisions of the nerve would effectively cause anesthesia, but the injection was painful. Furthermore, temporary motor weakness was common and pain relief was often only transient.
Thereafter, attention was turned to chemical destruction of the ganglion. In 1910, Wilfred Harris performed a completely percutaneous injection of alcohol into the trigeminal ganglion and cistern. In a large cohort of 1433 patients, he reported excellent rates of complete anesthesia and freedom from pain [76]. Glycerol was fortuitously found to be an effect neurolytic agent, since a combination of glycerol and tantalum dust was injected into the trigeminal cistern and used as a localization technique for stereotactic radiosurgery [77]. Chemical neurolytic techniques all inject the trigeminal cistern, which is a cerebrospinal fluid-filled space, and are not selective for particular divisions. Weakness of the muscles of mastication, anesthesia dolorosa, keratitis, and unilateral loss of taste were common if not ubiquitous. If alcohol spread out of Meckel’s cave, or if the needle was not at the precise target, then other cranial neuropathies would occur, potentially presenting with facial weakness, hearing loss, or oculomotor palsy. Glycerol rhizotomy is an effective procedure with respect to pain relief, but of all the surgical techniques in the treatment of trigeminal neuralgia, it has the highest incidence of both pain recurrence (54%) and of anesthesia dolorosa (approximately 2%) [78–83].
In order to minimize the risks of chemoneurolysis, the technique was refined over the following decades. Fritz Härtel described a percutaneous method of accessing the ganglion through the foramen ovale [84], and multiple clinicians reported the use of x-ray imaging to confirm accurate needle position [85, 86]. Today, the foramen ovale can be percutaneously targeted under fluoroscopic guidance with the assistance of external landmarks (Fig. 66.4). The skin is punctured at a point 2.5 cm lateral to the labial commissure. The operator inserts a finger into the mouth and touches the pterygoid process in order to guide the needle to the skull base. The target can be found at the intersection of two imaginary lines: the first being a horizontal line extending from the tragus to the tip of the nose, and the second being a vertical line extending down from the mid-pupil. Using lateral fluoroscopy, the needle is advanced until it meets the clival line, 5–10 mm below the sellar floor. Aspiration of cerebrospinal fluid confirms location in the trigeminal cistern. Alternatively, contrast media can be injected into the cistern and viewed on fluoroscopy.
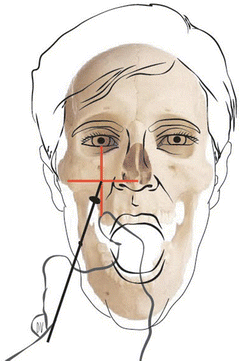

Fig. 66.4
Percutaneous trigeminal rhizotomy. The trigeminal ganglion and retroganglionic root in Meckel’s cave can be accessed percutaneously through the foramen ovale under fluoroscopic guidance. Rhizotomy may be performed with radiofrequency ablation, or more rarely now chemical gangliolysis or mechanical balloon compression. The skin is punctured 1–2.5 cm lateral to the labial commissure. The operator often places a finger in the mouth on the pterygoid process to guide the needle or cannula just lateral to this landmark. The target is initially determined by external landmarks: the intersection of a horizontal line at the level of the tragus and a vertical line at the mid-pupil. Fluoroscopy is used to access the foramen ovale and to determine the target site relative to the clival line and sellar floor
Sean Mullan and Terry Lichtor introduced mechanical balloon compression as an alternative to chemical destruction of the sensory root or ganglion in 1983 [87]. This technique was inspired by an older Taarnhøj-Sheldon-Pudenz procedure, in which the ganglion or sensory root was operatively exposed and deliberately compressed in order to elicit pain relief; however, recurrence rates were extremely high. In Mullan and Lichtor’s operation, general anesthesia is required as mechanical compression of the trigeminal ganglion can be excruciatingly painful. In the original description of the procedure, the foramen ovale is accessed percutaneously under fluoroscopic guidance. After cannulation of the foramen, a no. 4 Fogarty catheter with a 0.75 mL balloon is inserted into Meckel’s cave and is then inflated with 0.5–1 mL of contrast fluid for 5–7 min. A review of the major reports of balloon decompression [88–93] demonstrates excellent initial pain relief with an expected high incidence of facial numbness. However, the primary limitation of this technique is the prohibitively high incidence of trigeminal motor dysfunction (66%).
Thermal coagulation of the trigeminal ganglion is a superior alternative to chemical neurolysis or balloon compression. Additionally, it has been shown to allow for some degree of selection of a particular division. Initially, a monopolar current was applied to an insulated needle [94]. William Sweet and James Wepsic developed a novel approach whereby radiofrequency was used to thermocoagulate the preganglionic nerve fibers [95, 96]. By the time of their report in 1974, multiple advancements and adjunctive techniques had made this a much safer procedure. First, prior to any destructive procedure, they injected local anesthetic in order to create a temporary diagnostic nerve block, which facilitated adequate patient selection. Second, electrical stimulation was used to map out the area of putative lesion, which allowed for selection of a specific division of the trigeminal nerve and avoidance of the motor root. Third, a dual temperature monitoring probe was used concurrently with the application of radiofrequency, which allowed for control of the lesion size and avoided unintended damage to the motor root or first division. Altogether, these changes led to a lower incidence of loss of corneal reflex and subsequent keratitis .
Radiofrequency rhizotomy was at one time the most common procedure performed for trigeminal neuralgia. At least four reports can be found in the literature, which include cohorts of 1000 patients or more [97–100]. In particular, one pooled analysis of 6205 patients who underwent percutaneous radiofrequency ablation provides an excellent analysis of outcomes [101]. Initial and immediate pain relief was outstanding (98%) after radiofrequency ablation, and the result was durable; however, 20% of patients did have recurrence of pain. Nearly all patients had facial numbness, which was a correlate and marker of an efficacious procedure. This may be considered a potential disadvantage of the procedure compared to non-lesional treatments, such as microvascular decompression, yet <10% of patients have bothersome dysesthesias. Corneal anesthesia occurs in 7% of patients, but anesthesia dolorosa or keratitis occurs in only 1–2%. Major perioperative morbidity is low (1.2%), and there is virtually no mortality.

Full access? Get Clinical Tree





