Fig. 4.1
Definition of malnutrition
Insufficient energy intake
Weight loss
Loss of muscle mass
Loss of subcutaneous fat
Localized or generalized fluid accumulation that may sometimes mask weight loss
Diminished functional status as measured by handgrip strength
The influence of malnutrition on operative mortality and morbidity in general surgery (Studley 1936; Mullen et al. 1980; Gibbs et al. 1999; Stijn et al. 2013) and colorectal surgery patients (Schwegler et al. 2010; Lohsiriwat et al. 2008; Planas et al. 2007) has been extensively reported. Nutrients are crucial in the synthesis of proteins involved in the immune response to surgery and the inflammatory phase of wound healing (Haydock and Hill 1986; Scrimshaw and San Giovanni 1997; Rai et al. 2002). Malnutrition is a risk factor for overall complications, specifically nosocomial infections (Pessaux et al. 2003; Schneider et al. 2004) and impaired respiratory muscle function (Windsor and Hill 1988), leading to higher rates of surgical site infection and postoperative pneumonia.
Patients with malnutrition have significantly longer hospital stays, likely associated with an increased risk of complications and delayed recovery from such complications (Chima et al. 1997; Braunschweig et al. 2000; Middleton et al. 2001; Kyle et al. 2005; Pirlich et al. 2006; Kudsk et al. 2003b). The treatment cost of complications and the longer lengths of hospital stay in malnourished patients increase the economic burden of their hospitalization. The cost of treating a patient at risk of malnutrition is estimated to be 20–36 % higher than the average of the respective diagnosis-related group (Chima et al. 1997; Amaral et al. 2007). A study of patients in 25 Brazilian hospitals revealed a 60.5 % increase in daily expenses (US$138) for malnourished patients (Correia and Waitzberg 2003). Patients at nutritional risk were also more likely to require home health-care services or transfer to a transitional facility upon discharge.
Some groups have taken the opposite approach and analyzed the benefits of nutrition therapy on patients at risk of malnutrition. Adequate preoperative and postoperative nutrition therapy based on a standardized nutritional assessment model significantly decreased overall complications, major septic complications, and mortality in surgical patients (Mullen et al. 1980). The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group demonstrated fewer noninfectious complications in the severely malnourished patients treated with perioperative total parenteral nutrition (PN) (The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group 1991).
4.3 Transdisciplinary Clinical Nutrition Education and Nutrition Support Team (NST)
Despite the high occurrence and negative impact of malnutrition on clinical outcomes and hospitalization costs, the nutritional status of patients remains poorly identified and documented (Waitzberg et al. 2001; Singh et al. 2006; Gout et al. 2009). This highlights the need for a continuing education program in clinical nutrition for health-care providers, which should begin in medical and nursing schools (Acuna et al. 2008) and extend into residency and nursing training curricula.
Medical societies and quality improvement organizations have proposed structured guidelines to screen, identify, and treat malnutrition. Crucial to the successful implementation of these guidelines is the formation of nutrition support teams (Nehme 1980; Schneider 2006). The NST represents a model of transdisciplinary management where specialists from multiple disciplines combine their knowledge and expertise to achieve management decisions and outcomes superior to those obtained in isolation. An effective NST is a physician-led team of doctors, nurses, dieticians, and pharmacists who actively manage patients through daily clinical rounds using evidence-based protocols (Gales and Gales 1994). These teams are expected to interact closely with referring physicians, promote awareness, and improve the education of health-care providers in all aspects of nutrition care. The exact composition and roles of team members should be adapted to fit local demands and resources.
Successful NST practice has led to an increase in the appropriate use of PN and reduction in catheter-related complications and metabolic derangements (Nehme 1980; Gales and Gales 1994; Sriram et al. 2010). The implementation of an NST and nutritional clinical practice guidelines in a set of patients undergoing elective surgery for colorectal cancer resulted in a significant reduction in postoperative complications and hospital length of stay (Planas et al. 2007). It is important for each NST to determine performance goals or aims in terms of clinical nutrition outcomes of their patients. Regular audit of performance indicators will identify areas which require improvement or a change in clinical practice (Schneider 2006).
4.4 Clinical Pathway for Nutrition Risk Screening, Assessment, Therapy, and Monitoring
Nutrition screening is a simple process to identify patients at risk of malnutrition who will require a more thorough and detailed nutrition assessment to determine nutritional status (Kondrup et al. 2003). Many guidelines recommend screening in all cancer patients during the initial preoperation evaluation, either in the inpatient or outpatient setting. It is often challenging, however, to accurately measure nutritional status. Several markers have been explored as an indicator of nutritional status including albumin, prealbumin, and total lymphocyte counts (Elia 2000), but no single clinical or laboratory marker can be recommended as a comprehensive indicator of nutritional status. Levels of laboratory markers may be lowered during the acute phase response in inflammatory conditions making them unreliable in patients presenting with an acute colorectal problem (Fuhrman 2002).
In the 1990s, The Joint Commission mandated nutrition screening within 24 hours of admission, followed by a full nutrition assessment if found to be at nutritional risk. This guideline has since been widely adopted; however, colorectal patients who are undergoing elective procedures on the day of admission ought to be screened preoperatively in the outpatient clinic (Kudsk et al. 2003a). Nutrition screening tools should be easy to use, cost-effective, valid, reliable, and sensitive. Several nutrition screening tools have been described including the Mini Nutritional Assessment (MNA) (Vellas et al. 1999), the Malnutrition Universal Screening Tool (MUST) (Ferguson et al. 1999), and the Nutritional Risk Screening (Kondrup et al. 2003). The main limitations of these tools are the dependency on subjective parameters and clinical judgment and the variable evidence of their accuracy and reliability.
After nutrition screening, patients who are identified to be at risk of malnutrition should receive a more detailed nutritional assessment by a nutrition expert. This is a complete and systematic examination of the metabolic, nutritional, or functional variables which allows the NST to create an appropriate nutritional plan for each patient (Kondrup et al. 2003). ASPEN recommends using the Subjective Global Assessment (SGA), which is an assessment tool that consists of both the patient’s history and physical assessment (Fig. 4.2). Patients are subjectively classified as A (well nourished), B (moderate malnutrition), or C (severe malnutrition) (Detsky et al. 1987). The SGA is a validated and simple assessment that predicts postoperative complications with high accuracy (Baker et al. 1982).
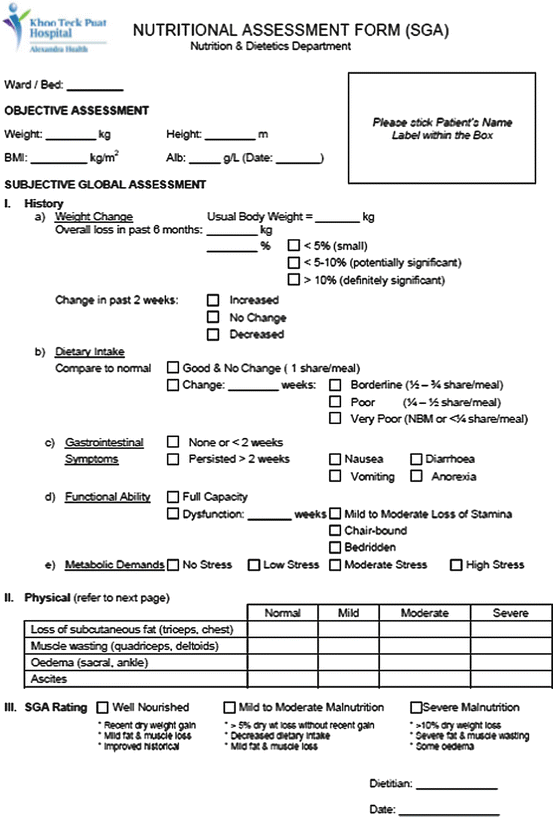

Fig. 4.2
Subjective Global Assessment
Patients assessed to be malnourished will require a nutrition therapy care plan, which should be delivered by a transdisciplinary team of nutrition experts, especially for those requiring PN. Regular reassessments of nutritional status must be conducted throughout the hospital stay to identify any changes in clinical or nutritional status, which may impact current nutrition status and therapy (Fig. 4.3).
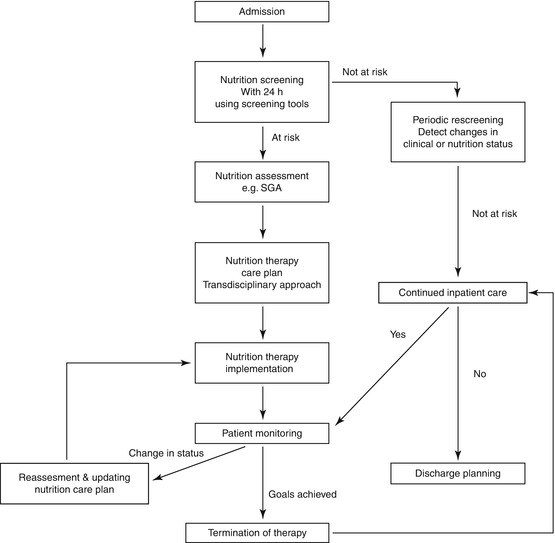

Fig. 4.3
Clinical pathway for nutrition risk screening, assessment, therapy, and monitoring
4.5 Calculating Nutritional Requirements
The crucial element in designing a nutrition therapy care plan is the calculation of a patient’s daily energy requirement. The daily requirement depends on resting energy rate, diet-induced thermogenesis, and physical activity (Manchanda 2003). The resting energy expenditure of patients after major colorectal surgery increased by 13 % compared to those in healthy volunteers, with no difference in urinary nitrogen losses (Soop et al. 2004). The change in the energy expenditure of cancer patients is variable and is dependent on body composition and tumor type and stage (Elia 2005).
Indirect calorimetry represents the gold standard for measuring energy expenditure in the clinical setting. This machine, however, is often unavailable due to its high cost. Most nutrition therapists use predictive equations to estimate energy expenditure; the components of these equations usually include characteristics which influence the amount of fat-free mass including body weight, height, age, and gender. Many equations have been developed for healthy individuals (Table 4.1) and can be adjusted for sick patients using stress factors (Frankenfield et al. 2004).
Table 4.1
Common energy expenditure equations
Equation (year) | Calculation |
|---|---|
Harris–Benedict (1919) | Men: Wt (13.75) + Ht (5) – age (6.8) + 66 |
Women: Wt (9.6) + Ht (1.8) – age (4.7) + 655 | |
Mifflin–St Jeor (1990) | Men: Wt (10) + Ht (6.325) – age (5) + 5 |
Women: Wt (10) + Ht (6.25) – age (5) – 161 | |
Penn State (1998, 2004, 2001) | Age ≥ 60 with BMI ≥ 30 kg/m2: Mifflin (0.71) + Tmax (85) + Ve (63) – 3,085 |
All others: Mifflin (0.96) + Tmax (167) + Ve (31) – 6,212 |
ESPEN recommends the use of 25 kcal/kg ideal body weight as an approximate estimation of daily energy requirements and 30 kcal/kg ideal body weight during severe stress. ESPEN guidelines also recommend 1.2–1.5 g/kg ideal body weight/day of protein to limit nitrogen losses in surgical patients (Braga et al. 2009).
In addition to calorie and protein requirements, patients should receive daily recommended amounts of fluids, electrolytes, trace elements, and vitamins (Tables 4.2, 4.3, and 4.4) (Langley 2007; Clark 2007).
Table 4.2
Daily electrolytes requirements, etiologies, and clinical consequences of abnormalities
Electrolyte | Recommended daily intake | Abnormal levels | Causes | Clinical consequences |
|---|---|---|---|---|
Sodium | 1–2 mmol/kg/day | Low | Replacement of lost solute with water | Malaise |
SIADH | Delirium | |||
Renal failure | Seizures | |||
Medications | Coma | |||
Respiratory arrest | ||||
High | Loss of water | Lethargy | ||
Iatrogenic (e.g., excessive use of hypertonic saline) | Irritability | |||
Seizures | ||||
Coma | ||||
Potassium | 1–2 mmol/kg/day | Low | Increased GI/GU losses | Muscle weakness |
Medications (e.g., insulin, nebulizers) | Ileus | |||
Carbohydrate loading | Cardiac arrhythmias | |||
Rhabdomyolysis | ||||
High | Increased intake | Muscle weakness | ||
Reduced urinary excretion (e.g., renal failure, medications) | Paralysis | |||
Cardiac arrhythmias | ||||
Magnesium | 4–10 mmol/day | Low | Impaired intestinal absorption | Tetany |
Increased renal excretion | Seizures | |||
Cardiac arrhythmias | ||||
Sudden cardiac death | ||||
High (rare) | Chronic kidney disease | Bradycardia | ||
Hypotension | ||||
Asystole | ||||
Calcium | 5–7.5 mmol/day | Low | Chronic kidney disease Hypoparathyroidism | Tetany |
Vitamin D deficiency | Hypoactive reflexes | |||
Alcoholism | Anxiety | |||
Hyperphosphatemia | Hallucinations | |||
Hypotension | ||||
High | Malignancy Hyperparathyroidism | Constipation | ||
Muscle weakness | ||||
Confusion | ||||
Lethargy | ||||
Phosphate | 20–40 mmol/day | Low | Increased renal elimination | Delirium |
Refeeding syndrome | Seizures | |||
Respiratory alkalosis | Respiratory failure | |||
Cardiac failure | ||||
Cardiac arrhythmias | ||||
High | Chronic kidney disease | Hypocalcemia | ||
Hypoparathyroidism | ECG changes | |||
Paresthesia |
Table 4.3
Daily trace element requirements, function, and clinical consequences of deficiency
Trace element | Recommended daily intake | Function | Deficiency |
|---|---|---|---|
Chromium | 10–15 mcg | Glucose and lipid metabolism | Rare |
Potentiation of insulin action Enhances tyrosine phosphorylation of the insulin receptor | Weight loss | ||
Neuropathy | |||
Impaired glucose tolerance | |||
Glucosuria | |||
Hyperlipidemia | |||
Copper | 0.3–0.5 mg | Oxidation–reduction and electron transfer reactions involving oxygen | Uncommon |
Component of metalloenzymes (used for collagen and elastin production, dopamine conversion to norepinephrine) | Defects in connective tissue | ||
Anemia | |||
Leukopenia | |||
Neutropenia | |||
Hypercholesterolemia | |||
Cardiac dysfunction | |||
Manganese | 60–100 mcg | Component of metalloenzymes (arginase, used for urea production; manganese superoxide dismutase, provides antioxidant function; pyruvate carboxylase, used in carbohydrate synthesis) | Rare |
Neurotoxicity | |||
Poor reproductive performance | |||
Abnormal bone and cartilage formation | |||
Ataxia | |||
Growth retardation | |||
Defects in carbohydrate and lipid metabolism | |||
Selenium | 20–60 mcg | Defense against oxidative stress | Keshan disease (cardiomyopathy in pediatric population) |
Regulation of thyroxine | Skeletal muscle disorders | ||
Regulation of the redox status of vitamin C and other molecules | |||
Zinc | 2.5–5 mg | Component of enzymes (RNA polymerase, alkaline phosphatase) | Growth retardation |
Structural role in protein folding | Hair loss | ||
Lipid peroxidation | Diarrhea | ||
Apoptosis, cellular proliferation, and differentiation | Delayed sexual maturation and impotence | ||
Immune function | Eye and skin lesions | ||
Loss of appetite | |||
Delayed wound healing | |||
Iron | 8–18 mg | Oxidative metabolism | Impaired physical work performance |
Heme proteins (hemoglobin, myoglobin, cytochromes) | Mental delay | ||
Electron transfer | Cognitive impairment | ||
Hypochromic–microcytic anemia |
Table 4.4
Daily vitamin requirements, function, and clinical consequences of deficiency
Vitamin | Recommended daily intake | Function | Deficiency |
|---|---|---|---|
A | 700–900 mcg | Bone growth | Night blindness |
Reproduction | Hyperkeratosis | ||
Cell division | Anorexia | ||
Immunity | Phrynoderma | ||
Cell differentiation | Depressed T-helper cell activity | ||
Impaired mucus secretion | |||
B1 (thiamine) | 1.1–1.2 mg | Coenzyme in metabolism of carbohydrates and branched-chain amino acids | Beriberi |
Wernicke’s encephalopathy | |||
Lactic acidosis | |||
B2 (riboflavin) | 1.1–1.3 mg | Coenzyme in numerous redox reactions | Ariboflavinosis (sore throat, hyperemia, and edema of pharyngeal and oral mucous membranes, cheilosis, angular stomatitis, glossitis, seborrheic dermatitis, normochromic–normocytic anemia) |
B3 (niacin) | 14–16 mg | Coenzyme in numerous redox reactions | Pellagra (pigmented rash, vomiting, constipation or diarrhea, bright red tongue, neurological symptoms including depression, apathy, headache, fatigue, and loss of memory) |
B5 (pantothenic acid) | 5–15 mg | Component of coenzyme A Component of the fatty acid synthase complex | Extremely rare |
Irritability and restlessness | |||
Nausea, vomiting, and abdominal cramps | |||
Numbness, paresthesia, muscle cramps, staggering gait | |||
B6 (pyridoxine) | 1.3–1.7 mcg | Coenzyme in the metabolism of amino acids, glycogen, and sphingoid bases | Seborrheic dermatitis |
Microcytic anemia | |||
Epileptiform convulsions | |||
B12 | 2.4–5 mcg | Cofactor for methionine synthase and L-methylmalonyl-CoA mutase which are essential for normal blood formation and neurologic function | Pernicious anemia |
Neurological manifestations (sensory disturbances in the extremities, motor disturbances, gait abnormality) | |||
Cognitive changes (loss of concentration, memory, disorientation, and frank dementia) | |||
Visual disturbances | |||
Insomnia | |||
Impotency | |||
Impaired bowel and bladder control | |||
C (ascorbic acid) | 200 mg | Antioxidant | Anorexia |
Biosynthesis of connective tissue components (collagen, elastin, fibronectin, proteoglycans, bone matrix, elastin-associated fibrillin), carnitine, and neurotransmitters | Fatigue | ||
Scurvy (anemia, bleeding gums, perifollicular hemorrhage, impaired wound healing) | |||
D | 2.5–15 mcg | Maintenance of normal blood levels of calcium and phosphorus | Osteomalacia |
Promotes bone mineralization | Osteoporosis | ||
Regulates cell growth, differentiation, immune function | |||
E | 15 mg | Antioxidant | Neuronal degeneration |
Plays role in immune function and in DNA repair | Platelet aggregation | ||
Inhibits cell proliferation, platelet aggregation, and monocyte adhesion | Decreased red blood cell survival | ||
Hemolytic anemia | |||
Skeletal muscle lesions | |||
K | 90–120 mcg | Coenzyme for synthesis of proteins involved in blood coagulation and bone metabolism | Rare |
Bleeding | |||
Folic acid | 400 mcg | Coenzymes involved in DNA synthesis, amino acid interconversions, single-carbon metabolism, methylation reactions | Hypersegmented neutrophils, macrocytic anemia |
Weakness, fatigue | |||
Difficulty concentrating, irritability | |||
Palpitations | |||
Shortness of breath |
4.6 Preoperative Management of Nutrition in Colorectal Surgery
4.6.1 Immediate Preoperative Management
Routine prolonged periods of fasting before surgery should be avoided. It is now known that consuming fluids up to 2 h before general anesthesia does not increase the risk of aspiration (Jones et al. 2011). There is also a lack of evidence to show harm when this is performed in patients at risk of reflux and gastroparesis, such as those with diabetes mellitus, gastroesophageal reflux disease, and obesity (Smith et al. 2011).
In addition, providing carbohydrate calories just before surgery in the form of a carbohydrate drink has been shown to shorten the length of hospitalization, reduce the loss of muscle mass, and possibly result in an earlier return of gut function (Jones et al. 2011; Noblett et al. 2006). Patients undergoing surgery in a fed state will not suffer from thirst or hunger. This reduces catabolism, maintains lean body mass, and reduces insulin resistance (Gustafsson et al. 2012; Ljungqvist and Søreide 2003). Therefore, patients undergoing colorectal surgery should continue to receive preoperative nutrition up to 2 h before the operation. This is given in the form of an oral carbohydrate drink on the day of the surgery (Smith et al. 2011). In diabetic patients, carbohydrate loading may be given with diabetic medication if necessary (Gustafsson et al. 2012; Nygren et al. 2012).
4.6.2 Preoperative Nutrition Therapy for Malnourished Patients
In the preoperative period, successful nutritional intervention aims to optimize caloric goals, improve nitrogen balance and lean body mass, and replenish depleted micronutrients in malnourished patients undergoing elective colorectal surgery. This involves the provision of fortified enteral or PN. Interventions may be divided into the provision of micro- and macronutrients, provision of immunonutrition, and reducing the period of fasting before surgery.
It may seem intuitive to optimize caloric goals and protein intake in every patient before surgery, but the benefits of delaying surgery to pursue nutritional optimization have only been shown in malnourished patients. Trials on preoperative nutrition optimization of malnourished patients have demonstrated reduced rates of surgical site infection, perioperative mortality, and length of hospitalization (Mullen et al. 1980; The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group 1991; Burden et al. 2011; Bozzetti et al. 2000). ESPEN, therefore, recommends preoperative nutritional support in severely malnourished patients. Nutritional support is given for 10–14 days before the operation. Surgery may be delayed if safe to do so, in order to optimize patients for the procedure.
4.6.2.1 Enteral Nutrition (EN)
EN is always preferred to PN as the former provides nutrition to enterocytes and is physiological and associated with less electrolyte, metabolic, and infective complications. Enteral formulas have less of an economic burden, and since EN does not require intravenous access, it is more cost-effective and easier to deliver in the outpatient setting (Bozzetti et al. 2001). The majority of surgical patients will achieve their nutritional goals with the addition of fortified oral supplements. If oral intake is still poor, then EN may be advanced with the use of a nasoenteral tube.
4.6.2.2 PN
If malnourished patients are unable to tolerate EN, e.g., partial bowel obstruction or high-output enterocutaneous fistula, PN should be given to optimize their nutrition. PN is recommended if the enteral intake is less than 60 % of the requirements and the anticipated duration of PN is more than 10 days or if the patient is unable to eat for more than 7 days (Braga et al. 2009).
4.6.2.3 Combined EN and PN
There is a paucity of data on combined enteral and PN in the preoperative period. Studies have concentrated on combined therapy in the postoperative critically ill patient, and this will be discussed in the postoperative nutrition section.
4.7 Postoperative Management of Nutrition in Colorectal Surgery
4.7.1 Nasogastric Decompression
Routine nasogastric tube decompression of the stomach after elective colorectal surgery is not recommended. Multiple meta-analyses have shown an increased risk of fever, atelectasis, and pulmonary infection and no benefit in anastomotic leak rates with the routine use of a nasogastric tube postoperatively (Cheatham et al. 1995; Nelson et al. 2005).
4.7.2 Postoperative Nutrition Therapy for Malnourished Patients
4.7.2.1 EN
Immediate oral intake after recovery from anesthesia is safe in elective surgery and is not associated with an increased risk of anastomotic dehiscence (Reissman et al. 1995; Han-Geurts et al. 2007; Lewis et al. 2009). The most recent meta-analysis suggested a reduction in morbidity and mortality and decreased length of hospital stay (Lewis et al. 2009). In malnourished patients, consumption of oral nutritional supplements postoperatively was associated with a reduction in chest and wound infections. This practice also led to improvements in both physical and mental quality of life measurements 18 months after surgery (Beattie et al. 2000).
If patients at nutritional risk are unable to consume an adequate amount of supplements via the oral route, enteral therapy can be provided via feeding tubes, e.g., nasoenteral or jejunostomy tubes. The use of these tubes is well tolerated and should be attempted before switching to PN (Braga et al. 2002). Since jejunostomy tubes are inserted at the time of the colorectal procedure, preoperative identification of patients at risk is required to optimize operative planning.

Full access? Get Clinical Tree








