Fig. 15.1
In the top row, the three schematics for gas chromatography show that samples containing morphine, hydrocodone, and a combination of the two can be distinguished from one another because the time it takes the compounds to elute from the system differ. In contrast, in the bottom row, the three schematics show that an immunoassay cannot distinguish among these samples. In each case, the region shaded more darkly represents a positive result; the region shaded less darkly, a negative result. In the leftmost figure, a sample with a morphine concentration of 300 ng/mL is right at the threshold for a positive result; a sample with a morphine concentration of 1000 ng/ml, positive. In the middle figure, because of hydrocodone’s reduced cross-reactivity, a hydrocodone concentration of 479 ng/mL is reguired to reach the positive thresold for the assay. In the right-hand figure, a sample containing both drugs would test positive, but one cannot know whether it represents only morphine, only hydrocodone, or a combination of the two
To be absolutely sure that another compound has not “co-eluted” at a given time, the compound(s) eluting at each time is (are) subjected to a second analysis, typically mass spectroscopy. In this technique, each compound presumptively identified by its retention time is ionized, and the resulting fragments, characteristic for the compound, are identified by their mass/charge ratios [7]. A typical mass spectrum for morphine is depicted in Fig. 15.2. If a compound other than morphine eluted at the same time by gas chromatography, it would have an entirely different mass spectrum.
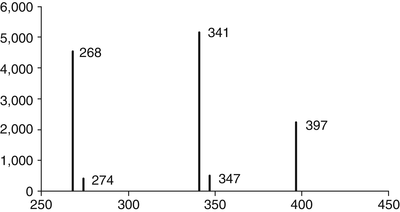

Fig. 15.2
Mass spectrum for morphine. After ionization, morphine is broken down into characteristic fragments, whose relative abundances are plotted against their mass/charge ratios. In this assay, the fragment with a mass/charge ratio of 341 is most abundant, followed by fragments with ratios of 268 and then 397; fragments with ratios of 274 and 347 are much less common. The pattern is unique for morphine
Based on the retention time as well as the mass spectrum, the drug’s identity can be assured. As will be seen in the examples later, this is a fundamental principle in clinical toxicology – one must identify each compound by two methods, each of which is based on a distinct analytical principle. It is possible that other compounds could co-elute and/or that other compounds might have the same (or similar) fragmentation patterns, but it is virtually impossible that another molecule would share both characteristics. These methods are the gold standards for identification, but they both are labor-intensive, demand significant expertise, and require expensive capital equipment.
It is relatively straightforward for the laboratory to determine whether its method can detect a given drug and the smallest concentration it can reliably detect. Thus, the laboratory should be able to provide its users with this data relatively easily. In addition, the laboratory should indicate which drugs are not in its repertoire, and it presumably would be able to offer advice on alternative ways to detect them. The laboratory report should explicitly indicate every individual drug for which the sample was tested, along with the detection limit. Thus, if the “opioid panel” does not explicitly indicate that testing for methadone (or tramadol, or buprenorphine, or fentanyl) was done, then the correct inference is that the test was not done. In most cases, none of these drugs is part of a typical opioid panel [8]. Too often, physicians infer that if a drug is not specifically mentioned, then it was not present.
As noted earlier, opiate screening immunoassays (which include techniques such as EMIT [9], FPIA [10], KIMS [11], CEDIA [12], etc.) can be, and are, performed by virtually any clinical laboratory, often in connection with an emergency toxicology program. Space does not permit a discussion of all the different methods here, but it may help to describe one method, one that is familiar to the author. The salient clinical performance characteristics are very similar for other methods.
In the EMIT assay [13], a small aliquot of a patient’s sample is added to a cuvette in which an antibody and an enzyme-labeled drug are present. The drug from the patient and the enzyme-labeled drug compete for the limited sites on the antibody molecules. When the enzyme-labeled drug molecule binds to the antibody, the enzyme activity is inhibited. The more drug present in the patient sample, the less of the enzyme-labeled drug is bound to the antibody (i.e., this is a competitive immunoassay), and therefore, the more enzyme activity remains in the cuvette [14]. Substrate for the enzyme is added, and the enzyme activity is measured spectrophotometrically as the slope of the line relating absorbance to time, as depicted in Fig. 15.1. If the slope of the line is greater than that of the calibrator (in this case, 300 ng/mL morphine), the result is considered positive; if it is lower, then the result is negative.
As shown in Fig. 15.1, though, the enzyme activity for hydrocodone and the enzyme activity for morphine cannot be distinguished from each other. Each drug with which the antibody reacts will cause an increase in enzyme activity, but it is the same enzyme, and therefore there is nothing unique about the reaction. Thus, one cannot tell from this test which drug is present. In addition, one cannot know if there is one drug, or more than one drug, present. Moreover, one cannot know how much of any given drug is present because the cross-reactivities of the antibody with each drug are not 100 % (see later). In contrast to the case with gas chromatography (even when it is not paired with mass spectroscopy), then, one cannot determine from opiate screening immunoassays which opioids are present nor how much of any drug is present. As a result, it is important, at least in the field of pain management, that positive screening immunoassays be subjected to further analysis to identify which opioids are present.
Another important limitation of opiate screening immunoassays is that a number of important opioids are not detected. Although each assay has its own characteristics, opioids typically not detected include methadone, meperidine, oxycodone, tramadol, buprenorphine, and fentanyl [7]. At this point, it might be worthwhile pointing out that, although people tend to use the terms “opiate” and “opioid” interchangeably, there is a distinction. “Opioids” is the term used to describe all drugs with morphine-like actions; “opiates” are those opioids derived from opium, a group that includes morphine and codeine. Thus, it is perhaps not coincidental that the screening immunoassays are referred to as “opiate immunoassays” rather than “opioid immunoassays”; they are particularly adept at detecting the drugs with structures similar to morphine (see later) [15, 16].
Figure 15.3 provides a flow chart suggesting one way to utilize screening immunoassays effectively in connection with pain management programs; the flow chart needs to be customized to the specific assays available from each laboratory.
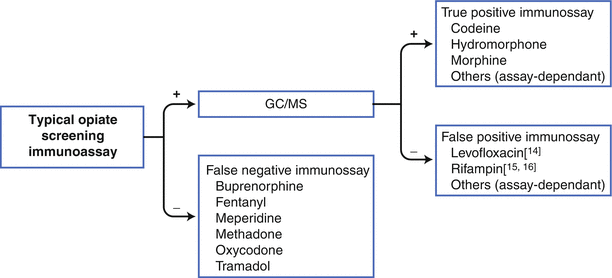

Fig. 15.3
Flow chart for using opiate immunoassays effectively. When using opiate immunoassays as the first step in screening urine samples, it is important to realize that several opioids may not be detected (buprenorphine, fentanyl, meperidine, methadone, oxycodone, and tramadol). Also, all positive results should be confirmed by a second method, such as gas chromatography/mass spectroscopy. If a specific opioid cannot be confirmed, then the original immunoassay result must be considered a false positive. As with all the examples in this chapter, the chart must be customized for each laboratory methods
Specimen of Choice
Physicians often wonder why laboratories prefer to do opioid measurements on urine, a preference that gives rise to other problems (e.g., sample collection and adulteration). When a blood sample is drawn, one is reasonably certain that one knows the identity of the patient from whom it came and that it has not been adulterated in any way, not to mention that one can always get a blood sample from a patient.
There are many reasons for the analytic preference for urine. Drugs are typically concentrated manyfold in the urine, and drugs are present in the urine in relatively high concentrations for many hours [17]. Even in the best case scenario, where one knows exactly which opioid was ingested and when the ingestion occurred, so that one can predict reasonably well when the blood peak concentration will occur, the peak value is manyfold less than the typical urine concentration. For morphine, as shown in Table 15.1, a 0.125 mg/kg intravenous injection will result in a peak concentration in blood of 440 ng/mL after 1 min, which will rapidly decline to just 20 ng/mL at 2 h [18] (and less than that at later times). In contrast, in a study where the peak serum concentration was 100 ng/ml (4.4 fold lower than that just mentioned), the urine concentration averaged 1, 568 ng/mL at 3 h and 720 ng/mL at 24 h [19]. The lesson is clear – if one is trying to detect morphine (or other opioids), it will be easiest to detect it in the urine.
Table 15.1
Urine versus serum morphine concentrations
Route (amount) | Serum peak (time) | Other serum (time) | Urine peak (time) | Other urine (time) |
|---|---|---|---|---|
Intravenous [18] (0.125 mg/kg, ∼9 mg) | 440 (0.5 min) | 20 (2 h) | ||
Intramuscular [18] (0.125 mg/kg, ∼9 mg) | 70 (15 min) | 20 (4 h) | ||
By mouth [18] (30-mg tablet) | 20 (4 h) | |||
By mouth [19] (7.5 mg from poppy seeds) | 100 (2 h) | 6 (24 h) | 1,568 (3 h) | 720 (24 h) |
Another reason that most clinical laboratories prefer urine samples is that the assays they use (screening immunoassays) are FDA-approved for use with urine samples only. Laboratories are permitted to run these assays on urine after doing relatively straightforward validation studies. In order to run these same assays on blood, however, laboratories are required to undertake much more extensive validation studies, studies that few laboratories have the resources to do.
Nonetheless, there are a few caveats about using urine samples that are important to keep in mind. As mentioned earlier, specimen collection can be problematic. In the absence of a discrete witnessed voiding, it is possible for patients to substitute pristine urine samples for their own, and it is also possible for patients to add adulterants to a sample of their own urine, adulterants which can interfere with some testing methods [20, 21]. Even with a valid sample from the correct patient, massive hydration, or skipping a drug for several days, can render the concentration so low that it becomes undetectable by some methods [2, 21]. In other words, it is difficult to document conclusively that a patient has not used a drug.
Concentration/Metabolites
As mentioned earlier, with screening immunoassays, it is never clear which opioids are causing the positive reaction; a positive reaction looks the same no matter which opioid caused it. In truth, it is even a little more complicated. Although each assay is calibrated to turn positive at 300 ng/mL of morphine, other opioids show different amounts of cross-reactivity (Table 15.2) [22]. This is true whether one considers a given manufacturer’s assay (columns) or whether one looks at a given opioid across manufacturers (rows). For example, for assay 1, hydrocodone will turn positive at a concentration of 100 ng/mL, but oxycodone will not turn positive until the concentration reaches 1,000 ng/mL; for assay 2, the corresponding figures are 479 and 23,156 ng/mL; and for assay 3, 364 and 5,388 ng/mL. Does this mean a sample with a concentration of 250 ng/mL of hydrocodone will be reported as positive by assay 1 and negative by assays 2 and 3? Absolutely!
Table 15.2
Immunoassay cross-reactivities
Assay 1 | Assay 2 | Assay 3 | |
|---|---|---|---|
Morphine | 300 | 300 | 300 |
Codeine | 50 | 225 | 247 |
Hydrocodone | 100 | 479 | 364 |
Hydromorphone | 100 | 620 | 498 |
Meperidine | 250,000 | 30,508 | >50,000 |
Oxycodone | 1,000 | 23,166 | 5,388 |
As mentioned earlier (and reflected in Table 15.2 for meperidine and oxycodone), none of the opiate screening immunoassays can be relied upon to detect buprenorphine, fentanyl, meperidine, methadone, or oxycodone. This is not entirely unexpected: these opioids have very different chemical structures, so one might predict that antibodies raised to morphine might not “recognize” them (Fig. 15.4).
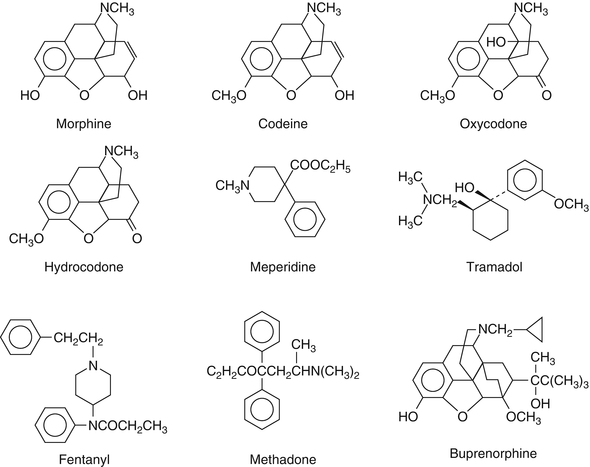

Fig. 15.4
Chemical structures of common opioids. The antibodies used in immunoassays are designed to detect morphine. One might predict that these antibodies would cross-react with codeine and hydrocodone, as their structures are so similar. By the same token, it is not surprising that these antibodies typically do not cross-react with meperidine, tramadol, fentanyl, methadone, and buprenorphine, whose structures are so different. Although, at first blush, oxycodone looks very similar to morphine, the presence of the (relatively large) hydroxyl (−OH) group in the center of the diagram must be sufficient to limit the cross-reactivity because few opiate immunoassays detect oxycodone (All structures were adapted from Magnani [22])
There is yet another layer of complexity with opiate screening immunoassays. For emergency toxicology purposes, it may be important to prevent false positives by setting the positive threshold at 300 ng/mL, at which concentration morphine is present in potentially clinically significant amounts. But for pain management physicians, the real question is whether it is there at all. Put differently, as just described, a patient whose urine has a hydrocodone concentration of 250 ng/mL will be reported as negative for opiates by assays 2 and 3, but a patient whose urine has a morphine concentration of 200 ng/mL will be reported as negative by all three assays! To make matters even worse, some laboratories are now using as their positive threshold for opiate screening immunoassays a concentration of 2,000 ng/mL [4, 23].

Full access? Get Clinical Tree





