Chapter 52 Thyroid emergencies
Thyroid emergencies are a rare cause for admission to critical care. However, mortality is high unless specific treatment is provided in an expeditious manner. Abnormal thyroid function tests are commonly encountered during critical illness; numerous factors must be considered before interpreting these findings as indicating thyroid disease.
BASIC PHYSIOLOGY
Thyroid hormones affect the function of virtually every organ system and must be constantly available for these functions to continue. The two biologically active hormones are tetraiodothyronine (thyroxine or T4) and triiodothyronine (T3). These are synthesised by incorporating iodine into tyrosine residues, a process which occurs in thyroglobulin contained within the lumena of the thyroid gland. Stimulation of hormone release by thyroid-stimulating hormone (TSH) results in endocytosis of thyroglobulin from the lumen into the follicular cells, followed by hydrolysis to form T4 and T3, which are released into the circulation.1
Both T4 and T3 contain two iodine atoms on their inner (tyrosine) ring. They differ in that T4 contains two further iodine atoms on its outer (phenol) ring, whereas T3 contains only one. T4 is produced solely by the thyroid gland whereas the majority of T3 is synthesised peripherally by the removal of one iodine atom (deiodination) from the outer ring of T4. If deiodination of an inner-ring iodine atoms occurs, the metabolically inert reverse-T3 (rT3) is formed. This is produced in preference to T3 during starvation and many non-thyroidal illnesses, and the ratio of inactive (rT3) to active T3 synthesis appears to play an important role in the control of metabolism.2 Numerous factors can affect the peripheral deiodination process (Table 52.1). Both T4 and T3 are highly protein-bound in the serum, predominantly to thyroid-binding globulin (TBG), but to a lesser extent to albumin and prealbumin. Changes in concentration of these serum-binding proteins have a large effect on total T4 and T3 serum concentrations. Such protein changes do not, however, affect the concentration of free hormone or their rates of metabolism. The serum-binding proteins act as both a store and a buffer to allow an immediate supply of the metabolically active free T4 (fT4) and free T3 (fT3). In addition, protein binding reduces the glomerular filtration and renal excretion of the hormones.
Table 52.1 States associated with decreased deiodination of thyroxine to triiodothyronine
The regulation of thyroid function is predominantly determined by three main mechanisms, the latter two providing physiological control. Firstly, availability of iodine is crucial for the synthesis of the thyroid hormones. Dietary iodide is absorbed and rapidly distributed in the extracellular fluid, which also contains iodide released from the thyroid gland and from peripheral deiodination processes. This becomes trapped within thyroid follicular cells, from which it is actively transported into the lumen to be oxidised into iodine and subsequently combined with tyrosine.3 Other ions, such as perchlorate and pertechnetate, share this follicular cell active transport mechanism, acting as competitive inhibitors for the process.
Secondly, thyroid hormone release is controlled by a closed feedback loop with the anterior pituitary. Diminished levels of circulating hormones trigger secretion of TSH which acts on the follicular cells of the thyroid gland, causing them to release thyroglobulin-rich colloid from the lumen, which is hydrolysed to form T4 and T3 for systemic release. Increased levels of T4 and T3 cause diminished TSH secretion, resulting in the follicular cells becoming flat and allowing increased capacity for colloid storage. As a result, less thyroglobulin is mobilised and hydrolysed with less T4 and T3 release. The degree to which TSH is secreted in response to changes in circulating thyroid hormones is dependent on the hypothalamic hormone thyrotrophin-releasing hormone (TRH), which is itself modulated by feedback from the thyroid hormones. TRH secretion is inhibited by dopamine, glucocorticoids and somatostatin.
THYROID CRISIS (THYROID STORM)
Thyrotoxic storm is arguably the most serious complication of hyperthyroidism, with reported mortality ranging from 10 to 75% in hospitalised patients.4,5 Crisis most commonly occurs as a result of unrecognised or poorly controlled Graves disease; however, other underlying diseases may be the cause.6,7 Females outnumber males. Laboratory findings are inconsistent due to acute disruption of the normal steady state of the circulating hormones, and there is no definitive value that separates thyrotoxicosis from thyroid storm. The latter is a clinical diagnosis and a scoring system has been proposed to guide the likelihood of the diagnosis (Figure 52.1).8 Precipitating factors are not always present, though many have been identified (Table 52.2).
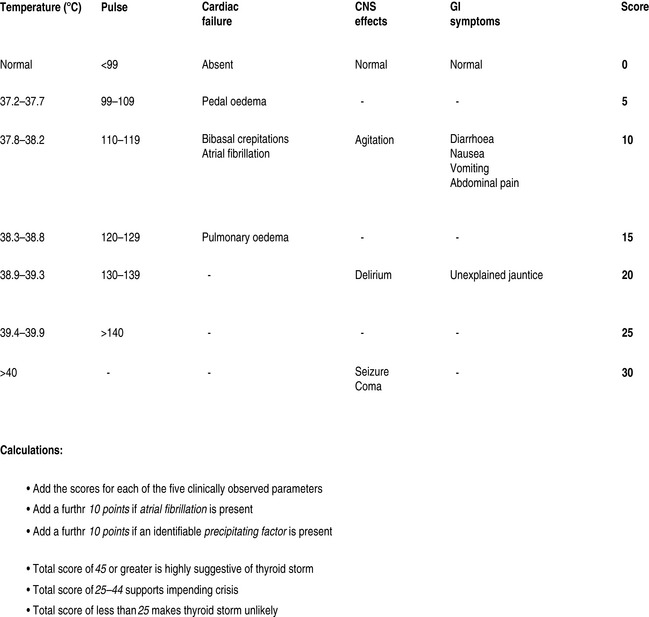
Figure 52.1 Severity assessment in thyroid crisis. CNS, central nervous system; GI, gastrointestinal.
(Adapted from Tewari K, Balderston KD, Carpenter SE et al. Papillary thyroid carcinoma manifesting as thyroid storm of pregnancy: case report. Am J Obstet Gynecol 1998; 179: 818–19.)
Table 52.2 Factors associated with precipitating thyroid storm
CLINICAL PRESENTATION (Table 52.3)
The classic signs of thyroid crisis include fever, tachycardia, tremor, diarrhoea, nausea and vomiting.9 However presentation is extremely variable and may range from apathetic hyperthyroidism (apathy, depression, hyporeflexia and myopathy)10 to multiple-organ dysfunction.11,12 Differential diagnosis includes sepsis and other causes of hyperpyrexia such as adrenergic and anticholinergic syndromes.
Table 52.3 Clinical features of hyperthyroidism/thyroid crisis
FEVER
This is the most characteristic feature. Temperature may rise above 41°C. There have been suggestions that pyrexia is present in all cases of thyroid storm,13 though normothermia has been reported.11 Pyrexia is rare in uncomplicated thyrotoxicosis and should always raise suspicion of thyroid storm. It is not clear whether this febrile response is due to alteration of central thermoregulation or elevation of basal metabolic thermogenesis beyond the body’s ability to lose heat.
CARDIOVASCULAR FEATURES
Fluid requirements may be substantial in some patients, whereas diuresis may be required in those with severe cardiac congestion. Cardiac decompensation can occur in young patients with no known antecedent cardiac disease. Systolic hypertension with widened pulse pressure is common initially; however hypotension supervenes later. Cardiogenic shock with vascular collapse is a preterminal sign.14 Arrhythmias are common and include atrial and polymorphic ventricular tachycardias. The latter may result from prolonged QTc interval, the presence of which may complicate and contraindicate the use of amiodarone therapy.
NEUROMUSCULAR FEATURES
Tremor is a common early sign, but as ‘storm’ progresses, central nervous dysfunction evolves with progression to encephalopathy or even coma.15 Thyroid storm has been reported in association with status epilepticus and cerebrovascular accident.16 Weakness may be a feature, particularly with apathetic thyrotoxicosis.10 Thyrotoxic myopathy and rhabdomyolysis may be present,11,17 the latter being differentiated from the former by its association with markedly elevated creatine phosphokinase levels. A number of other syndromes of neuromuscular weakness have been described, including hypokalaemic periodic paralysis18 and myasthenia gravis.19
GASTROINTESTINAL FEATURES
Diarrhoea, nausea and vomiting are common, though the patient may present with symptoms of an acute abdomen.20 Severe abdominal tenderness should raise the possibility of an abdominal emergency. Liver function tests may be abnormal due to congestion or necrosis and tenderness over the hepatic area may be present. Hepatosplenomegaly may be present. The presence of jaundice is a poor prognostic sign.14
LABORATORY FINDINGS
Numerous abnormalities may be found:
MANAGEMENT
β-ADRENERGIC BLOCKADE
This is the mainstay of controlling adrenergic symptoms.21 Intravenous propranolol titrated in 0.5–1-mg increments, while monitoring cardiovascular response, diminishes the systemic hypersensitivity to catecholamines. In addition it inhibits peripheral conversion of T4 to T3.22 Concurrent administration of enteral propranolol is the norm, with doses as high as 60–120 mg 4–6-hourly often being necessary due to enhanced elimination during thyroid crisis.23 An alternative regimen uses intravenous esmolol with a loading dose of 250–500 μg/kg followed by infusion at 50–100 μg/kg per min. This allows rapid titration of β-blockade while minimising adverse reactions.24 β1-selective antagonists such as metoprolol may be of use, particularly in the presence of reactive airways and heart failure. These agents do not inhibit conversion of T4 to T3 to the extent of non-selective β-antagonists and should be combined with other therapy. Patients who exhibit resistance to β-adrenergic blockade may be successfully treated with reserpine or guanethidine, though their onset of action is slow and side-effects may be significant.

Full access? Get Clinical Tree













