
THROMBOEMBOLIC DISEASE
Venous thromboembolism (VTE) disease, the composite term for deep vein thrombosis (DVT) and pulmonary embolism (PE), is a complication that deserves a prominent place in the mind of any surgeon caring for acutely ill or injured patients. As concepts of rapid triage and resuscitation accomplish their goals of optimizing the numbers of surgical patients surviving their initial physiologic insult, an ensuing challenge is represented by the attendant risk of these potentially lethal complications disproportionally seen in those who are immobilized and recuperating. The specter of the problem has reached sufficient magnitude to prompt the U. S. Congress to designate March as DVT Awareness Month1 and the Surgeon General in 2008 to issue “A Call to Action to Prevent Deep Venous Thrombosis and Pulmonary Embolism.”2
This chapter discusses VTE from the standpoint of incidence, risk factors, and screening philosophy and impact on “quality.”
Virchow’s triad composed of venous stasis, endothelial injury, and hypercoagulabilty, is the well-known classic explanation for the basic underlying pathway toward DVT. Once DVT occurs, there are many different natural outcomes that may follow. At one end of the spectrum, the body’s normal fibrinolytic system can prevent propagation and lyse the clot, potentially even before the patient becomes symptomatic. At the other end of the spectrum would be the case of a large DVT traveling through the vascular system and causing sudden death from a large saddle pulmonary embolus. Other frequent scenarios include DVT that are clinically apparent with significantly swollen extremity and the potential for long-term sequelae of postthrombotic syndrome and/or PE that become symptomatic with pleuritic chest pain, shortness of breath or hypoxia requiring immediate treatment.3
Multiple patient populations treated by acute care surgeons are at exceedingly high risk of VTE. ICU admission is often suggested as an independent risk factor for VTE, although those patients quite likely also have multiple other well-accepted risk factors. In a study of 110 mechanically ventilated ICU patients, nearly 25% were diagnosed with DVT when the group was liberally screened with duplex ultrasound, in spite of appropriate VTE prophylaxis. In addition, 11.5% of patients with DVT in this study were diagnosed with PE as well.4 In trauma patients, VTE remains an important clinical problem,5 with the DVT rate as high as 58% if only high risk patients are considered in the risk pool and aggressive diagnostic surveillance is applied.6 However, the reported rates are often lower than 1%, especially when registry data are used.7–9 Within the field of general surgery, historical data, from the years before VTE prophylaxis was used, sheds some light on the underlying risk of VTE in certain populations. Within the group of untreated general surgery patients, the risk of DVT and PE were 19.1% and 1.6%, respectively. The rate for fatal PE was a remarkable 0.87%.10 Even in the current era in which we frequently use prophylaxis, VTE rates of over 2.5%–3.5% are reported after some major general surgery and surgical oncology cases.11
Many different factors can increase a patients’ risk of developing VTE.12 Once a patient has VTE diagnosed, he/she is always at elevated risk for recurrent VTE, especially if provoked by an acute event or new diagnosis. These varied risk factors can be categorized by the patient’s primary diagnosis category (i.e., trauma, cancer), other medical conditions (i.e., congestive heart failure), treatments performed (i.e., central line placement), hypercoaguable state (hereditary or acquired), or other predisposing conditions (i.e., obesity, advanced age). Some of the commonly accepted risks are listed in Table 55.1. In addition, these risk factors seem to be additive such that the more risk factors an individual patient has, the higher their risk of VTE.12,13 Many procedures commonly performed on acute care surgery patients are also associated with elevated VTE occurrence rates including central line placement (femoral is highest risk location), major surgical procedures, chemotherapy administration, and fracture immobilization. Nearly every diagnosis that increases the odds of VTE by >10-fold is commonly treated by the acute care surgeon: fracture (hip or leg), major general surgery, major trauma, and spinal cord injury12,13 (see Table 55.2). Additional factors specific to VTE risk in trauma patients are included in Table 55.3.
TABLE 55.1
RISK FACTORS OBSERVED IN 1,231 CONSECUTIVE PATIENTS TREATED FOR ACUTE DVT AND/OR PE
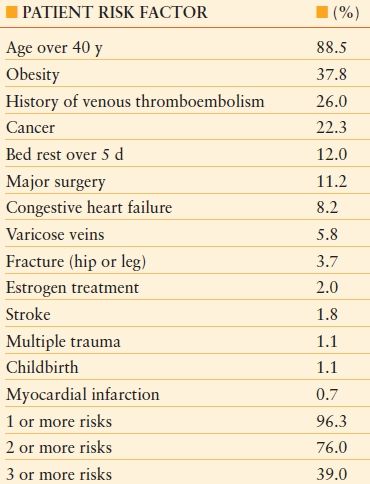
Modified from Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1): I9–I16.
TABLE 55.2
RISK FACTORS FOR VTE
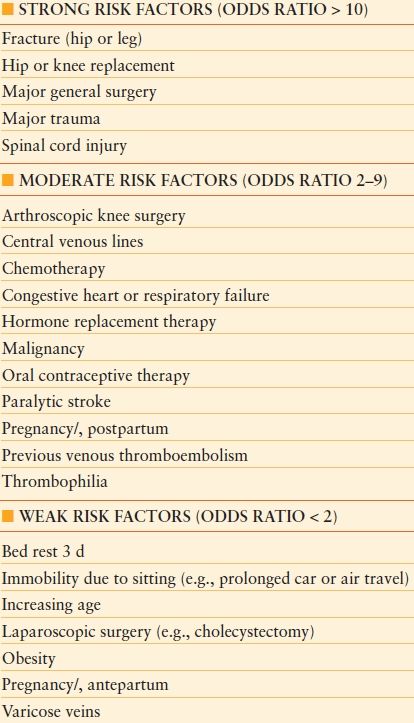
Modified from Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1): I9–I16.
In addition to clinical impact on individual patients affected by VTE, there are significant financial implications of VTE occurring both during and following hospitalization. In one study of orthopedic surgery patients, mean hospital length of stay was more than doubled for patients with VTE compared to those without VTE. The study also reported the mean total costs of inpatient care nearly twofold higher for the VTE patients.14 A study of cardiovascular surgery patients had similar findings; VTE patients had 14% higher inpatient costs and 68% longer length of stay as compared with those patients not having a VTE.15 The economic burden of having DVT or PE as a secondary diagnosis during hospitalization is estimated to be $7,594 and $13,018, respectively.16 In addition, this same study showed a high rate of readmission (14%) for patients with a secondary diagnosis of DVT or PE.16
VTE PROPHYLAXIS
According to the Agency for Healthcare Research and Quality, VTE prophylaxis in the appropriate patient populations is a top safety priority for hospitalized patients. Evidence-based guidelines published by specific specialty groups (e.g., The Eastern Association for the Surgery of Trauma [EAST]17 and The American College of Chest Physicians [ACCP])18 utilize risk stratification to suggest which patients are at what level of risk and allow for appropriate suggested protocols for VTE prophylaxis.
The attached risk stratification tool (Algorithm 55.1) and algorithm-based prophylaxis regimen is one example for trauma patients that takes into account several concepts from the surgical literature: (1) enoxaparin, a low molecular weight heparin (LMWH), demonstrates an efficacy advantage over unfractionated heparin in a prospective randomized trial with trauma patients with ISS ≥ 9. (2) Normal ambulation (when appropriate) is an excellent adjunct in prophylaxis. (3) No regimen is 100% effective to prevent all VTEs,19 suggesting some role for diagnostic surveillance in some high risk patients.20
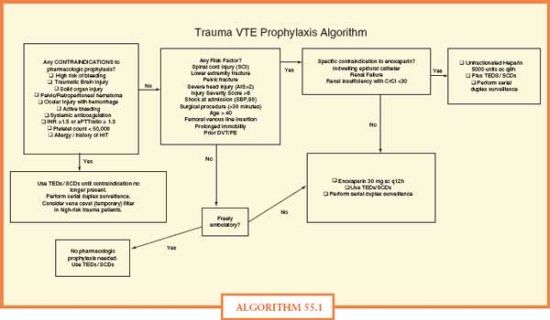
ALGORITHM 55.1 Algorithm.
Prophylaxis, in general, is divided into two main categories. The first is chemical or pharmacologic prophylaxis, including unfractionated subcutaneous heparin or LWMH given prophylactically to patients. In surgical patients, there is also some proven benefit that the preoperative dose, given 1–2 hours before surgery, is important to help with the prevention of clots. Although there maybe some associated elevated risk of bleeding with the prophylaxis, this small elevated risk is far outweighed by the substantial drop in symptomatic VTE events and PE-related death. In the current era, nearly all admitted surgical patients will have at least some risk factors warranting pharmacologic prophylaxis. Extended prophylaxis (beyond hospitalization) is also recommended for some surgical populations treated by acute care surgeons—specifically those undergoing cancer surgery21–24 and major abdominal and pelvic surgery.25,26
Mechanical prophylaxis includes sequential compression devices, intermittent pneumatic compression devices, and graduated compression stockings. These mechanical devices also have a benefit in reducing the risk VTE and are especially helpful in patients who have a specific contraindication to chemical prophylaxis and cannot get either unfractionated or LMWH. Even with the accepted improvement in VTE rates with mechanical devices, studies have shown they cannot be used in some trauma patients due to wounds, casts, immobilizers, or external fixators27 and that compliance with these devices on surgical patients is poor even without any specific contraindications.28
In orthopedic surgery specifically, there are multiple other types of prophylaxis that has been recommended. There are very good data that long-term prophylaxis for up to 6 weeks with warfarin in patients after hip replacement surgery has been shown to decrease event rates for both symptomatic DVT as well as PE.18 It is suggested that aspirin may be used for prophylaxis in certain orthopedic patient populations, although there is still much ongoing debate, and many hematologists and thrombosis experts believe that this is likely inappropriate.
The prophylactic use of IVC filters for prevention of PE in patients without proven DVT remains controversial. The use of these filters in trauma patients who are deemed to be of very high risk for PE has increased in recent years in the hopes that these prophylactic inferior vena cava filters can prevent massive PE and sudden cardiac death.29 There is no consensus on exactly what patient population such filters should be used in, although in clinical practice they are placed for this specific indication in many trauma patients every year. There are temporary IVC filters that can be safely deployed by vascular and/or trauma surgeons in addition to interventional radiologists with a plan to remove them after the period of highest risk has ended. However, there is still ongoing concern that even with the planned removal of the devices, many filters remain in situ as trauma patients often lack appropriate follow up to have the filters taken out.30
Even with the large amount of strong evidence suggesting prophylaxis against VTE, the routine use of acceptable prophylaxis is surprisingly low both in the United States and around the world.31–34 In surgical patients, rates of guideline-compliant prophylaxis often hover around 50%. In an attempt to increase this low compliance with best practice prophylaxis, many approaches have been taken35 including computerized decision support36 and electronic alerts.37
Even as recently as 20 years ago, DVT was a difficult diagnosis to make because the test, contrast venography, was difficult to perform, invasive, painful, and likely caused more DVTs at a later date. However, presently, the duplex ultrasound is now the well-accepted standard of care for diagnosis of DVT.17 This relatively inexpensive, noninvasive test has also likely increased the overall numbers of DVTs identified since it can be looked for more easily, even in the patient with a relatively low index of suspicion.
The Diagnosis of Pulmonary Embolism
Historically, before the modern era of diagnostic imaging, many pulmonary emboli were identified only at autopsy and were not suspected premortem. The earliest test for diagnosing a PE was pulmonary arteriogram, which was an invasive technique performed by interventional radiologists or cardiologists, whereby, contrast was injected directly into the pulmonary artery via right heart catheterization. Invasive pulmonary arteriogram gave way to the V/Q or ventilation/perfusion mismatched scan, upon which many of the major early studies regarding diagnosis of PE (i.e., PIOPED study) were based.38
Currently, computed tomography (CT) pulmonary angiogram is clearly the most commonly performed test to identify PE, and this test has proved appropriate for the majority of patients except for patients who cannot get IV contrast due to renal dysfunction or allergy to the contrast material for whom ventilation-perfusion scanning remains an option.39 In addition, patients need to travel to the CT scanner for this diagnostic test, which can also be a problem for the hemodynamically unstable patient in the ICU. In this patient population, echocardiogram has been suggested to be the ideal test of choice. Echocardiogram findings of a dilated right heart and pulmonary hypertension are relatively specific for PE in the hemodynamically unstable patient. Serum testing of D-dimer levels serves as an excellent adjunct role in the diagnosis (specifically to help rule out DVT/PE) in the outpatient and emergency department settings.39 However, in the acute inpatient setting, D-dimer levels are frequently elevated in postsurgical patients as well as in those who are systemically ill, making this test less useful.
Treatment
The mainstay of treatment for patients with all VTE is medical in the form of anticoagulation. Even as recently as just a few years ago, treatment was always in a hospital setting. Patients would receive days of intravenous heparin drip with a bridge period until they were therapeutic on their warfarin, the major vitamin-K antagonist oral anticoagulation. However, there has been a major paradigm shift in that patients with VTE are now routinely treated with either once or twice a day dosing of LMWH, and then bridging that to warfarin, which can be safely performed on an outpatient in many patient populations. It is important to remember that when a patient is started on warfarin, there must always be an overlap period with some heparin (either unfractionated or LMWH) since there may be a short period of hypercoagulability immediately after vitamin K antagonists, that is warfarin, are given. Other anticoagulants can be used specifically in patients with a history of or active heparin-induced thrombocytopenia. In this case, direct thrombin inhibitors are utilized for treatment of DVT and/or PE. Many suggest that in these complex cases, consultations with specialists such as a hematologist who are well versed in the wide range of anticoagulants are to be implemented.
Duration of Treatment for Patients with VTE or PE
Although there has been recent debate on the needed length of therapy for DVT/PE, new evidence-based guidelines from the American Heart Association have attempted to streamline expert opinions. Length of therapy should be short (3 months) for those with a first DVT episode provoked by a major risk factor. Longer treatment (at least 6 months) is suggested for patients with recurrent or unprovoked DVT. It remains unclear exactly which patients should be screened for inherited hypercoagulable states (i.e., Factor V Leiden, antithrombin deficiency, protein C or S deficiency). However, if one is identified, this may change the suggested length of therapy. There are suggestions that repeat duplex ultrasound to confirm resolution of the clot is beneficial before stopping the anticoagulation.
Some hospitalized patients with DVT/PE cannot receive first line treatment with anticoagulation due to specific contraindications. These will include recent neurologic or ophthalmologic surgery, trauma patients with solid organ injury and/or retroperitoneal hematoma), preexisting coagulopathy or thrombocytopenia, or those at significant risk for recurrent falls. In these patients, IVC filter placement is the classically accepted treatment to prevent PE, although it will not help treat the underlying DVT.
Patients with PE and hemodynamic instability present a difficult treatment decision. Fibrinolysis, or thrombolytic therapy (i.e., Alteplase), is suggested for the treatment of massive PE in patients without contraindications. This treatment may break the PE into smaller pieces and allow better blood flow to potentially resuscitate the patient, such that they can move on to the next step of therapy. If thrombolytic is going to be given, it should be done immediately as soon as possible once the diagnosis of PE is either identified definitively or in a patient with cardiac arrest and significant risk factors. In patients with submassive PE, the evidence is less clear and clinical judgment is warranted to balance the risk/benefit ratio.40
In patients with submassive PE, there may be a role for either catheter-directed therapies such as embolectomy/fragmentation (usually performed by vascular interventional radiologists) or potentially an open surgical pulmonary artery thrombectomy (Trendelenberg procedure). The performance of this procedure is beyond the scope of this book, as it will routinely be performed by a cardiothoracic surgeon, through either a median sternotomy or anteriolateral thoracotomy. The decision to proceed down one or the other paths should be made in team consultation and will depend on local expertise with these procedures.40
SCREENING PHILOSOPHY AND QUALITY
Since injured patients are at elevated risk for DVT, many authors suggest that high-risk asymptomatic patients be screened for lower extremity DVT using duplex ultrasound. However, other authors have suggested that this process may not be cost effective as it has not been proven to improve patient outcomes. Recently published data show wide variability in trauma surgeons’ opinions and trauma center practices regarding the use of screening duplex ultrasound in asymptomatic trauma patients.20 Our policy of aggressive screening is driven by a desire to identify VTE as a DVT rather than a PE; however, recent federal government health policy decisions prompts reconsideration of this approach. In this section, we offer constructive criticism of the Center for Medicare and Medicaid Services’ (CMS) decision to treat DVT as a “never event”41 and the attendant thrust to use hospital DVT incidence rates as a marker for quality of care.19
We have previously reported evidence for surveillance bias in DVT reporting on a national level using the National Trauma Data Bank (NTDB), as well as at a single institution.
Our first exploratory analysis of the hypothesis that DVT rates reported may be related to rates of screening asymptomatic patients for DVT with duplex ultrasound was a retrospective review of trauma registry and hospital discharge data from a single academic level 1 trauma center.42,43 Patients admitted to the Adult Trauma Service were divided into two groups, those admitted before versus after implementation of a written guideline for DVT prophylaxis and duplex ultrasound screening of asymptomatic high-risk patients. Data were compared between these two groups. Significantly more duplex ultrasound exams were performed in the later period (20.9 vs. 81.5 per 1,000 trauma admissions, p < 0.0001). This outcome was expected based on the implementation of our new guideline. The proportion of patients with DVT diagnosed increased 10-fold from 0.7 to 7.0 per 1,000 admissions (p = 0.0024). The incidence of PE or mortality did not change significantly, but proportionately more VTEs were identified as DVTs rather than PEs.
Our next study was a hospital-level analysis of duplex ultrasound and DVT rates in the largest trauma database available, the NTDB.44 We divided hospitals into quartiles based on their rates of duplex ultrasounds per trauma patient admission and compared the DVT rates reported from hospitals in each quartile. Hospitals in the highest quartile by duplex rate reported a DVT rate sevenfold higher than the average DVT rate in the first three quartiles (1.52% vs. 0.22%, p < 0.001) (see Fig. 55.1). One plausible explanation for the large disparity in DVT and ultrasound rates between quartiles was practice variation between hospitals in screening asymptomatic trauma patients. The DVT rate increased significantly and correlated with duplex ultrasound rate at hospitals that had a 2% or greater ultrasound rate.
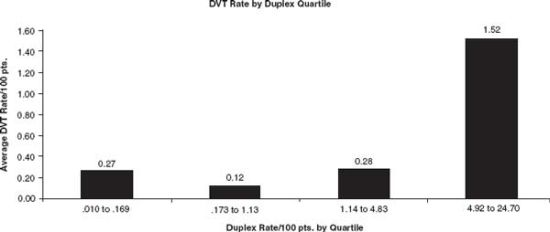
FIGURE 55.1. Hospital DVT rates in each quartile of hospitals. Raw data of the average hospital DVT rate per 100 admitted patients by duplex rate quartile. From Pierce CA, Haut ER, Kardooni S, et al. Deep vein thrombosis surveillance patterns in the National Trauma Data Bank: the more we look, the more we find. J Trauma. 2008;64: 932–937.

Full access? Get Clinical Tree








