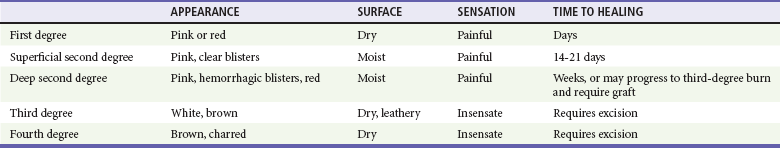
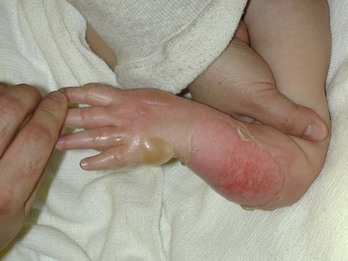
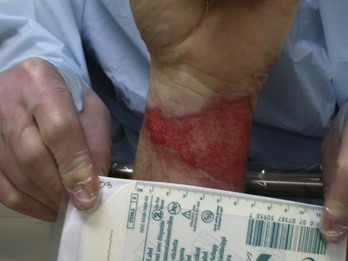
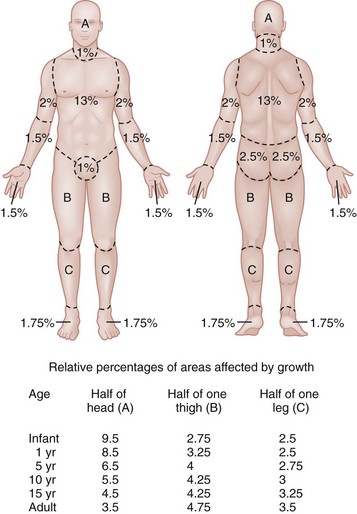
Chapter 63 Burns are relatively common injuries caused by direct or indirect contact with heat, electrical current, radiation, or chemical agents. The common denominator in all burns is protein denaturation and cell death. With thermal burns, the degree of injury is dependent on the temperature, the duration of the exposure, and the intrinsic structure of the burned tissue that determines its heat conductivity.1–3 Temperatures below 44° C are generally tolerated for prolonged periods without causing burns. As the temperature rises, there is an exponential decrease in the time to produce injury, because the proportion of unfolded protein molecules increases dramatically at supraphysiologic temperatures.4 At temperatures above 60° C, most tissue proteins are denatured. The proteins most predisposed to thermal denaturation are the lipid bilayer and membrane-bound adenosine triphosphatases. Thermal alteration of the plasma membrane is likely to be the most significant cause of the tissue necrosis.4 Cell death occurs by one of two mechanisms.5 Necrosis (or oncosis) is a passive process characterized by cellular swelling, depletion of intracellular energy stores, and massive release of inflammatory mediators. In contrast, apoptosis is a highly programmed, active process characterized by shrinkage and condensation of cell contents, DNA fragmentation, and budding without cellular swelling and a minimal inflammatory response. Both necrosis and apoptosis play a role in burn injury progression, although their relative role may vary.6,7 The underlying mechanisms leading to cellular necrosis and apoptosis are discussed further in the section on pathophysiology. According to the American Burn Association (ABA), 450,000 burn injuries are treated in medical facilities each year.8 This includes 3500 deaths, which occur mostly in residential fires. Of the 45,000 burn admissions each year, more than 55% are admissions to specialized burn centers. The majority of burns occur from fire or flame (44%), scalds (33%), contact with hot objects (9%), electricity (4%), or chemical agents (3%). More than one third of admissions (38%) exceed 10% total body surface area (TBSA), and 10% exceed 30% TBSA. Most admissions include severe burns of such vital body areas as the face, hands, and feet. The overall survival rate from burns in the years 1995 to 2005 was 94.4%.9 The National Center for Health Statistics indicates a decrease in the number of burn visits from 1996 to 2000, possibly because of improved fire prevention strategies, with no further changes in trends from 2000 to 2005.9 Half of burn patients in the emergency department (ED) are age 19 to 44 years. The most commonly affected body areas are the upper extremities (41%), lower extremities (26%), and head and neck (17%). Most of the burns are partial-thickness injuries, and less than 5% are full thickness. Unlike mechanical injuries, in which the amount of tissue damage is determined at the time of injury, burns are dynamic injuries that often progress in depth and size over the first few days after injury. Traditionally the burn injury has been divided into three concentric zones.10 At the center of the burn is a zone of irreversible coagulative necrosis that is formed immediately. Surrounding this central core is a zone of ischemia in which there is a reduction in the dermal microcirculation, putting this area at risk for subsequent necrosis if the perfusion is not improved. The zone of ischemia is the focus of intense research, directed toward reversing or preventing the conversion from ischemia to necrosis. The third and outermost zone of injury is the zone of hyperemia, characterized by an immediate and transient increase in perfusion. The ability of the skin to regenerate itself without scar formation is highly dependent on the level of injury.11 Regeneration of the damaged epidermis occurs from two primary sources. The basal layer of cells from the uninjured adjacent epidermis can give rise to reepithelialization of the burn; however, this is limited to 1 cm from the wound edges. With large burns the major source of regenerative epidermal cells comes from the dermal skin appendages: hair follicles and sebaceous glands. More damage to the epidermal appendages increases the likelihood of scar formation. Although the exact mechanisms that drive burn injury progression are unknown, several processes are thought to play a role.12,13 The inflammatory response results in the release of a large number of toxic cytokines, mediators, and free oxygen and nitrogen radicals that contribute to further injury.14–16 Occlusion of the dermal microcirculation with a combination of red blood cells, neutrophils, and microthrombi results in reduced perfusion and tissue ischemia that is exacerbated by a large amount of tissue edema.17 Cytokines, such as tumor necrosis factor alpha, result in cellular apoptosis and further inflammation; free oxygen and nitrogen radicals damage vital proteins, lipids, and DNA. Cellular membranes are especially vulnerable to oxidative stress caused by lipid peroxidation.18 The importance of these mechanisms is demonstrated by the ability of several inhibitors of inflammation and oxidative stress to reduce burn injury progression both in animals and humans.19–22 Unfortunately, none of these agents have demonstrated clinical utility. A predisposition to infection, metabolic derangements, and other factors also predispose partial-thickness burns to progress to deeper burns.13 Lack of adequate fluid resuscitation and hypoperfusion contribute to the deepening of the burn wound. Impaired ability to combat infection is common in patients with large burns and appears to be cytokine mediated. In particular, interleukin-12 (IL-12) has been shown to depress immune function.23 Abnormal complement activity also predisposes to infection in large burns. Exposure to heat can cause significant injury and rapidly developing edema to the upper airways in up to one third of burn patients with inhalation injury.24 However, owing to effective heat dissipation, direct thermal injury is generally not seen below the vocal cords.25 Other components of smoke, such as products of incomplete combustion (e.g., carbon monoxide [CO], cyanide, the aldehydes and oxides of sulfur and nitrogen), and particulate matter often result in significant ventilation-perfusion ( The upper and lower airway pathology is secondary to airway edema and de-epithelialization of the injured tracheobronchial mucosa, with progressive shedding of the necrotic lining of the airway and the formation of pseudomembranous casts that partially or completely obstruct the airway. These casts consist of necrotic epithelium, mucus, cellular debris, fibrinous exudate, polymorphonuclear leukocytes, and clumps of bacteria. Damage to the mucociliary function of the endobronchial mucosa reduces the ability to eliminate excess mucus and secretions. In addition, the pulmonary parenchyma becomes infiltrated with leukocytes that release inflammatory mediators and reactive oxygen species that further contribute to bronchospasm, tissue inflammation, and destruction. In particular, the local formation of nitric oxide (NO) results in vasodilatation and reversal of hypoxic pulmonary vasoconstriction, further aggravating The increase in extravascular water content and pulmonary lymph flow causes a marked decrease in the compliance of the lungs. Inhalation injury also results in deactivation of pulmonary surfactant, leading to areas of microatelectasis causing further ventilation perfusion mismatch and pulmonary shunting, and results in progressive hypoxemia and ultimately the clinical syndrome of acute respiratory distress syndrome.29,30 Ventilator-induced barotrauma further contributes to tissue injury and the release of toxic mediators. Classification and Diagnosis of Burns Burns are classified by mechanism of injury, depth, extent, and associated injuries and comorbidities. Determination of the depth of injury is crucial, as healing potential and appropriate treatments are contingent on the depth of injury (Table 63-1). The current burn classification system was first described by the French barber surgeon Ambroise Paré several hundred years ago. First-degree burns are limited to the epidermis and characterized by erythema and pain. They generally heal within several days to a week. Second-degree, or partial-thickness, burns extend through the epidermis and into the dermis. They may be divided into superficial and deep partial-thickness injuries. Superficial second-degree burns are limited to the superficial (papillary) dermis. The skin is erythematous and forms blisters, and the surface can be moist (Fig. 63-1). The wound blanches on pressure and is painful. Deep second-degree burns extend through the epidermis into the deep (reticular) dermis. They appear white with some erythematous areas with less blanching and moisture than the superficial second-degree burns (Fig. 63-2). The distinction between superficial and deep second-degree burns is important because deep second-degree burns do not heal within 2 to 3 weeks and often result in severe scarring and contractures, especially in children. Deep second-degree burns that do not heal within 21 days require excision and skin grafting to minimize scarring. Deep second-degree burns may also progress to third-degree burns over the course of several days after injury. Third-degree burns extend through the entire dermis and are referred to as “full-thickness” burns. Their appearance is stiff and white or tan. They are dry or may have a charred appearance (Fig. 63-3). They do not blanch and, because of destruction of the nerves, are not painful. Finally, fourth-degree burns extend through the skin and subcutaneous fat into the underlying muscle and bone. Fourth-degree burns are stiff and charred and have visibly thrombosed vessels. Most burns are not uniform in depth and have areas of varying depth of injury. Injection of radioactive isotopes and vital dyes, thermography, photometry, nuclear imaging, and ultrasound have been used to help determine burn depth and predict the potential of burns to heal.31 Currently the most promising tool is laser Doppler imaging; however, even this tool has little predictive value in the early stages in the ED. As a result, clinical evaluation in the ED is required to estimate burn depth. The accuracy of non–burn specialists in predicting burn depth is approximately 50%,32 and even among burn specialists the accuracy is only 75%.33 This emphasizes the need for close follow-up and serial assessments to distinguish between deep and superficial burns, as the injury is dynamic and often continues to evolve after initial presentation. The extent or surface area of the injury must be evaluated. Estimation of the percentage of TBSA burned is often inaccurate on initial inspection, and clinical rules and charts are used for more precise estimation of the percentage of TBSA.34–36 Careful estimation is critical because the volume of fluid administered is based on this evaluation and an overestimation of the extent of injury can lead to overzealous fluid administration.37,38 In addition, the criteria for referral to a burn unit are dependent on the surface area of the burn. Only second-degree and deeper burns should be used to calculate the percentage of TBSA affected. A useful clinical rule for smaller burns or scattered areas of burn is that the area of a patient’s palm and fingers is approximately 1% TBSA.39 For larger burns, the “rule of nines” is useful. This rule states that the allocation of percentage of TBSA in an adult patient can be estimated as follows: 18% for the front and 18% for the back of the trunk, 18% for each lower extremity, 9% for the head and 9% for each upper extremity, and 1% for the perineal area. A Lund-Browder chart is useful for estimating the extent of burns in children (Fig. 63-4).40 The rule of nines should not be used for children as their head is larger and, in proportion, their extremities are smaller than those of adults. Finally, burns are classified by severity into minor, moderate, and severe based on the TBSA burned, the percentage of full-thickness injury, and the involvement of specific areas such as the face, hands, feet, or perineum (Table 63-2). In addition, the presence of inhalation injury, high-voltage electrical burns, associated major trauma such as fractures, extremes of age, and comorbid conditions is considered. Burns should be covered with a clean dressing. Cooling with tap water or a commercially available cooling blanket helps reduce pain.41,42 Care should be taken to minimize hypothermia, especially when ambient temperature is low. Topical antimicrobial agents and greasy ointment should not be used during prehospital care because they offer few benefits during transport, must be removed when the patient arrives at the hospital, and may obscure accurate determination of burn depth. Management of the upper airway in burn patients may be extremely challenging. After exposure to superheated steam and toxic fumes, rapid and progressive upper airway edema may develop within minutes. If there is doubt regarding upper airway compromise, fiberoptic laryngoscopy should be performed. Endotracheal intubation guided by fiberoptic laryngoscopy or bronchoscopy is a useful technique, and if attempts at intubation are unsuccessful, surgical cricothyrotomy or needle cricothyrotomy may be necessary. After placement of an endotracheal tube, several devices are available to help secure it.43 With circumferential neck burns, the stiff burn eschar may compress the airway as well as the major neck vessels and require laterally placed escharotomies on either side of the neck, with extreme care taken to avoid damage to blood vessels and other underlying vital structures. Smoke inhalation injury affects 5 to 35% of hospitalized burn patients. With improvements in fluid resuscitation, inhalation injury has become one of the two major causes of mortality in burn patients, and the presence of inhalation injury more than doubles the mortality rate in adults.44,45 Traditionally, inhalation injury was diagnosed based on clinical findings such as facial burns, singed nasal vibrissae, carbonaceous sputum, and a history of injury within a closed space. However, these findings are neither highly sensitive nor highly specific.46 Similarly, wheezing, crepitations, hypoxemia, and abnormalities on the initial chest radiograph may or may not be present in the ED, except in the most severely injured. The diagnosis of inhalation injury in the ED is best made by direct visualization of the airways with fiberoptic laryngoscopy before intubation and bronchoscopy after intubation.47 Findings of inhalation injury include the presence of soot, charring, and mucosal inflammation, edema, or necrosis. Bronchoscopy can identify injury to the airway, but diagnosis of injury to the parenchyma of the lung is best made with xenon ventilation scanning, which demonstrates areas of decreased alveolar gas washout secondary to small airway obstruction. Xenon scanning is rarely used in the ED. CO levels should be measured in serum via CO-oximetry. Noninvasive devices that measure CO levels are now available in some ambulances and hospitals; these devices expedite recognition of CO poisoning. Cyanide, a frequent combustion product of plastics, should be suspected in patients injured in a closed-space fire who have elevated CO and lactate levels. (See Chapter 159.) Deciding when mechanical ventilation is required is critical. In addition to securement of the upper airway, there are several other indications for endotracheal intubation and mechanical ventilation (Table 63-3). Alveolar lavage may be required to help remove thick secretions and improve pulmonary toilette. This has been simplified by smaller and more flexible bronchoscopes.46 Table 63-3 Indications for Endotracheal Intubation and Mechanical Ventilation in Burn Patients There are many modes of mechanical ventilation, each having its own advantages and disadvantages. In the ED most patients who are intubated will require heavy sedation with or without paralysis, and therefore the assist control mode, in which the ventilator cycles automatically at a preselected rate, is most appropriate. Rapid sequence induction is the preferred method unless airway obstruction is suspected, in which case awake intubation with sedation may be necessary. The goals of mechanical ventilation should include achieving an acceptable oxygen saturation (>92%) and limiting the plateau airway pressures to 35 cm H2O or less to reduce the incidence of barotrauma.48 This may require allowing the partial pressure of carbon dioxide (PCO2) levels to rise to 35 to 55 mm Hg (permissive hypercapnia) as long as the pH remains greater than 7.25. A recent randomized trial found that low tidal volumes (6 mL/kg of predicted weight) reduced mortality.49 Starting tidal volumes should therefore be set at 6 to 8 mL/kg of predicted body weight. Larger tidal volumes may be required if oxygenation is not adequate. Positive end-expiratory pressure may also help oxygenation. For hyperoxic lung injury to be minimized, oxygen concentrations should be titrated to the lowest concentrations that maintain adequate oxygenation. A sample of initial ventilator settings is presented in Table 63-4. High-frequency percussive ventilation that administers high-frequency, time-cycled, pressure-limited subtidal volumes may reduce the incidence of barotrauma.50 Continuous positive airway pressure (CPAP) and BL-PAP may be considered in alert and cooperative patients who are difficult to oxygenate.51 However, endotracheal intubation should not be delayed in patients who fail to rapidly improve with noninvasive ventilation. Airway pressure release ventilation that uses CPAP applied at a high level with intermittent releases of airway pressure has also been used to help promote alveolar recruitment while reducing the risk of barotrauma.52 Table 63-4 Recommended Initial Ventilator Settings I/E, inspiratory-expiratory; PEEP, positive end-expiratory pressure.
Thermal Burns
Perspective
Epidemiology
Principles of Disease
Pathophysiology of Inhalation Injury
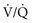 ) mismatch and pulmonary edema.26–28 (See Chapter 159.)
) mismatch and pulmonary edema.26–28 (See Chapter 159.)
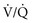 mismatch.28 NO combines with superoxide (O2−), which is released by neutrophils to form peroxynitrite (ONOO−). This reactive molecule then damages DNA and activates poly(adenosine diphosphate [ADP] ribose) polymerase, which is an enzyme that repairs damaged DNA while depleting the cell of large amounts of adenosine triphosphate (ATP), leading to necrosis.27–29
mismatch.28 NO combines with superoxide (O2−), which is released by neutrophils to form peroxynitrite (ONOO−). This reactive molecule then damages DNA and activates poly(adenosine diphosphate [ADP] ribose) polymerase, which is an enzyme that repairs damaged DNA while depleting the cell of large amounts of adenosine triphosphate (ATP), leading to necrosis.27–29
Clinical Features
Management
Emergency Department Management of Burns
Airway Management
Recognizing Inhalation Injury
Management of Inhalation Injury
Tidal volume
6-8 mL/kg
Respiratory rate
8-12 in adults
12-45 in children
Plateau pressures
<35 cm H2O
I/E ratio
1 : 1 to 1 : 3
Flow rates
40-100 L/min
PEEP
8 cm H2O

Full access? Get Clinical Tree


Thermal Burns
Only gold members can continue reading. Log In or Register to continue