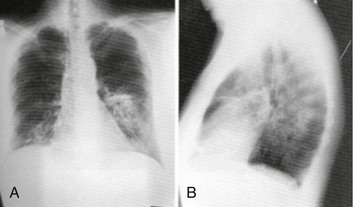
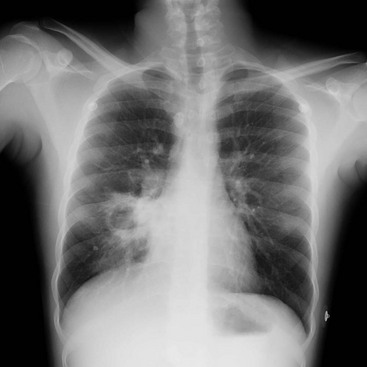
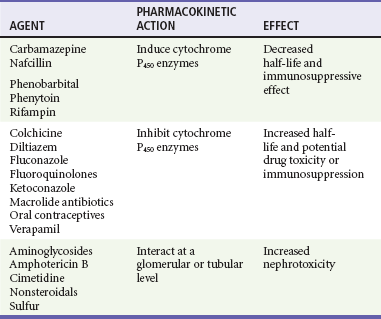
Chapter 184 Lifelong immunosuppression is generally necessary for all recipients of solid organ transplants, and infection is the primary cause of mortality after transplantation. Two thirds of transplant patients have at least one significant infection, most commonly nosocomial during the surgical recovery period or, subsequently, community acquired (Box 184-1).1,2 Signs of infection in this population of patients are often blunted by an impaired inflammatory response. Minor complaints or the chief complaint of a fever in an afebrile patient may herald a severe infection. Aggressive management usually translates into increased patient survival and graft function.1 Infections occurring in the first month after transplantation are likely to be related to the transplant procedure, stents, catheters, and intubation. In addition, the typical causes of postoperative fever should always be considered. Hospital-acquired pathogens are prominent, and management is similar for any immunosuppressed patient recently discharged.2 CMV is the most important and prevalent immunomodulating virus 1 to 6 months after transplantation.1 CMV infection may produce disease in multiple systems, but pneumonitis is particularly common and may be manifested insidiously. CMV infection may be primary or due to reactivation from latent viral particles found in lymphocytes. Typically, signs of CMV infection occur at a median of 40 days after transplantation. Survival from CMV infection is improving because of aggressive use of bronchoscopy that leads to earlier diagnosis and treatment with ganciclovir and CMV-specific immune globulin. Prophylaxis with ganciclovir reduces CMV incidence and death due to infection, but routine use of ganciclovir is associated with a number of potential side effects.3 Because the risk of CMV infection is greatest during antilymphocyte therapy, some transplant centers administer ganciclovir only selectively during the treatment period, although CMV infection is still often fatal.4,5 Active CMV infection can trigger or exacerbate organ rejection. CMV is linked to a particular form of glomerulopathy in renal allograft recipients as well as acute hepatic dysfunction and the disappearing bile duct syndrome of chronic hepatic allograft dysfunction.6,7 Furthermore, both acute cardiac dysfunction and accelerated coronary artery atherosclerosis of chronic heart transplant rejection are linked to CMV.8,9 EBV infection causes clinical effects similar to those of CMV infection. EBV contributes to immunosuppression and can cause a mononucleosis-like syndrome associated with lymphadenopathy, weakness, and low-grade fevers. Because CMV and EBV often coexist, both seem to trigger graft rejection. EBV is also implicated in B-cell lymphoproliferative syndrome, which is histologically similar to polymorphic B-cell lymphoma.10 Healthy Transplant.: Healthy transplant patients have no chronic immunomodulating viral infections and a functioning allograft; they are maintained with low doses of immunosuppressant medications. They have a mildly increased susceptibility to normal community-acquired infections, such as influenza, urinary tract infection, and pneumococcal pneumonia. Chronic Viral Infection.: Progressive disease may develop as a result of the combination of viral immunomodulating infections and long-term immunosuppression. Progressive liver disease due to recurrent or acquired viral hepatitis as well as hepatocellular carcinoma may occur. B-cell lymphoproliferative disorder associated with EBV infection may also develop.1,2 Primary varicella infection may lead to rapid dissemination with pneumonia, pancreatitis, hepatitis, encephalitis, and disseminated intravascular coagulation. Patients who are varicella-zoster virus (VZV) seronegative need high doses of intravenous varicella-zoster immune globulin after any exposure, preferably before the development of the rash. Therapy can be lifesaving if it is administered early before dissemination occurs.1,2 Reactivation of latent VZV infection, manifested as cutaneous herpes zoster, affects at least 10% of solid organ transplant recipients. Fortunately, reactivation of VZV is usually confined to a single dermatome and does not disseminate. Intravenous acyclovir therapy accelerates healing but does not change the incidence of painful neuralgia. Facial zoster involving the cornea and disseminated infections in more than one dermatome are indications for admission.1 In patients who have never contracted VZV, many patients are immunized before transplantation. Herpes simplex virus (HSV) reactivation is common after solid organ transplantation and can be manifested as oral or anogenital lesions, more often ulcers than vesicles. Some transplant centers prescribe oral acyclovir for 3 to 6 months after transplantation, whereas others choose to treat HSV immediately at the first sign of recurrence.11 Chronic Rejection.: Patients with chronic rejection require ongoing aggressive immunosuppressive treatment to protect the allograft. These patients are at the highest risk for life-threatening opportunistic infections with fungi (e.g., Candida, Cryptococcus, Coccidioides, Blastomyces, and Histoplasma), bacteria (e.g., Listeria and Nocardia), and parasites (e.g., Pneumocystis, Toxoplasma, and Strongyloides). Fungal infections are distinct to geographic regions and typically are manifested with subacute respiratory complaints associated with fever and focal, disseminated, or miliary infiltrates on the chest radiograph.1 Invasive candidiasis and aspergillosis can also develop in the chronically immunosuppressed group. Primary infections in the lungs, gastrointestinal tract, or nasal sinuses lead to fungemia and contamination of intravenous lines and wounds. Treatment is with antifungal agents such as amphotericin B or agents such as micafungin (Mycamine). Diverticulitis is the principal bacterial gastrointestinal infection observed in transplant patients with chronic rejection. Inflammation is suppressed with chronic steroid therapy, which leads to an increased incidence of perforation before diagnosis. Patients complain of an insidious onset of pain, change in bowel habits, and sometimes fever, but they typically lack peritoneal signs. Transplant patients are also susceptible to mucosal invasion with salmonellae (nontyphoidal) and Listeria. Infection with both organisms can be manifested as acute diarrheal syndromes that progress to bacteremia or meningitis.1,2 Pneumocystis jiroveci (carinii) pneumonia is often found in combination with CMV infection because CMV inhibits alveolar macrophage function (Fig. 184-1). Fortunately, its incidence is reduced with prophylactic low-dose trimethoprim-sulfamethoxazole. Without prophylaxis, infection may develop 1 to 6 months after transplantation and is manifested subacutely with fever, nonproductive cough, progressive dyspnea, and an interstitial infiltrate on the chest radiograph. Differentiation from CMV pneumonia is impossible without bronchoscopic confirmation. Treatment of Pneumocystis pneumonia includes intravenous trimethoprim-sulfamethoxazole and steroids, depending on the degree of hypoxia. Mycobacterial disease can be manifested as opportunistic reactivation or a primary infection. Even disseminated disease can be clinically silent, with invasion of the bowel, skin, and gastrointestinal tract. Pulmonary disease can be absent, miliary, or cavitary (Fig. 184-2). Treatment is difficult because typical agents can cause graft dysfunction.1,2 Rejection typically occurs in three phases: hyperacute, acute, and chronic. Hyperacute rejection is rare with careful donor-recipient matching. It typically occurs in the immediate perioperative period. Acute rejection occurs within the first months after transplantation. The patient presents with constitutional symptoms and signs of transplant organ insufficiency. Expeditious laboratory assessment, including possible allograft biopsy, can confirm the diagnosis of rejection, and an appropriate adjustment can be made in the immunosuppressant regimen. If immunosuppression is stopped, acute rejection may occur at any time. Chronic rejection has a time course of years and results in the gradual failure of the transplanted organ over time.12 Pharmacology of Immunosuppression A new term, operational tolerance, reflects long-term acceptance of transplanted organs without indefinite immunosuppression.13 Allogeneic bone marrow transplantation from the solid organ donor may facilitate operational tolerance. Successful bone marrow transplantation may revolutionize the treatment of solid organ rejection and immunosuppressive therapy. Accommodation, an acquired resistance of an organ to immune-mediated damage, has been recognized for more than 20 years. Initially, it was identified in blood group–incompatible kidney transplants that survived and functioned normally in recipients with high titers of anti–blood group antibodies directed against antigens in the grafts. Variable sublethal graft injury induces accommodation, and that may be a dynamic condition, eventuating into tolerance on the one hand and chronic graft injury on the other.14 Because of the toxicities of current immunosuppression medications, improvement in long-term transplantation outcomes may depend on new agents with novel mechanisms of action, devoid of the toxicities.15 Inhibitors of pathways in B-cell and plasma cell activation, including belimumab and atacicept, combat the humoral immune response. Biologic agents for maintenance immunosuppression include monoclonal antibodies and fusion receptor proteins that target molecular pathways and enzymes. Cyclosporine.: A variety of immunosuppression strategies are being tested, but the mainstay of transplant immunosuppression is cyclosporine.16,17 Cyclosporine inhibits both cellular and humoral immunity by binding to cyclophilins, which block cytokine transcription and production, thereby inhibiting lymphocyte signal transduction. The result is a potent immunosuppression of helper-inducer T cells, without affecting the suppressor T-cell subset.16,18 The helper-inducer T cells enhance antibody recognition and production by B cells. Cyclosporine exhibits dose-related nephrotoxicity, which is additive to other nephrotoxic drugs typically used after transplantation, such as amphotericin B, aminoglycosides, and high-dose trimethoprim-sulfamethoxazole. Cyclosporine causes renal tubular injury and direct renal artery vasospasm in a dose-dependent manner, leading to systemic hypertension in many recipients.16 Hypertension should be treated aggressively with standard regimens to preserve renal function and to prevent atherosclerosis. Cyclosporine may also aggravate hyperlipidemia, leading to atherosclerosis.19 Therefore, monitoring of cyclosporine levels and renal function is routine. Cyclosporine also causes hyperuricemia and gout in transplant patients. The nephrotoxic effects of cyclosporine complicate the management of gouty attacks in renal transplant patients.20 Traditionally, nonsteroidals are the mainstay of gout therapy, but their use is limited in transplant patients receiving cyclosporine because of a potential synergistic nephrotoxic effect. Cyclosporine levels are altered by many common post-transplantation medications (Table 184-1). Drugs that inhibit cytochrome P450 metabolism, such as erythromycin and ketoconazole, produce elevated cyclosporine levels and enhanced toxicity.21 Treatment with rifampin can increase cyclosporine metabolism, which triggers episodes of organ rejection. Cyclosporine can also cause hepatotoxicity, hyperkalemia, hirsutism, tremor, and gingival hyperplasia.16
The Solid Organ Transplant Patient
Principles of Disease
Infection
First Month after Transplantation
1 to 6 Months after Transplantation
6 Months after Transplantation
Rejection
Drug Toxicity of Immunosuppression
< div class='tao-gold-member'>

Full access? Get Clinical Tree