Class
BMI (kg/m2)
Underweight
<20.0
Normal range
20.0–24.9
Obese class 1
25.0–29.9
Obese class 2
30.0–34.9
Obese class 3
35.0–39.9
Obese class 4
40.0–49.9
Obese class 5
50.0–59.9
Obese class 6
≥60.0
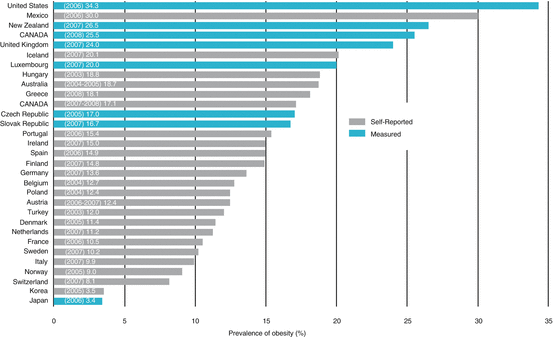
Fig. 12.1
Prevalence of obesity in OECD countries, 2004–2008. Recent obesity estimates indicate that measured obesity ranges from 10.5% in France, 25.5% in Canada, and up to 34.3% in the United States. From: Obesity in Canada. A joint report from the Public Health Agency of Canada and the Canadian Institute for Health Information. 2011
12.3 Comorbidities Associated with Obesity
12.3.1 Metabolic Syndrome
Obesity is further classified according to fat distribution, namely, the android and gynecoid obesity (Fig. 12.2). This distinction has important clinical implications [5].
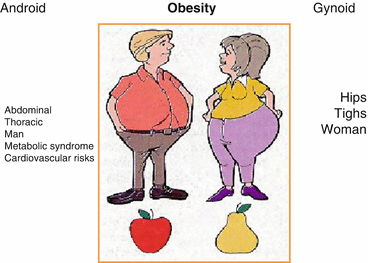

Fig. 12.2
Types of obesity related to gender. The fat distribution is more abdominal and thoracic in man (android obesity), while it is found on hips and thigh in woman (gynoid obesity). From: Prise en charge de l’obèse dans Anesthésie et réanimation, Ed Fellahi, 2014
Android (or central) obesity mainly involves the upper part of the body and often implies intra-abdominal visceral fat accumulation. This type of obesity predominates in men and can lead to the metabolic syndrome, a group of risk factors known to predispose to type 2 diabetes and cardiovascular disease. Indeed, from a physiological standpoint, adipose cells should not be regarded only for its role in energy balance and storage. Abdominal fat does participate in a prothrombotic and pro-inflammatory cascade that affects fatty acid metabolism and contributes to the development of insulin resistance [6]. There are several definitions of the metabolic syndrome, but recognition is mostly based on the finding of at least three of the following five criteria: (1) glucose intolerance, (2) abdominal obesity, (3) increased blood pressure, (4) low high-density lipoprotein (HDL) levels, and (5) increased triglyceride (TG) level [7]. The metabolic syndrome may have a negative impact on outcomes after noncardiac and cardiac procedures. It increases the risk of perioperative and postoperative complications [8]. In contrast, women more often present with a gynecoid (or peripheral) obesity that involves predominantly the accumulation of fat at the thighs and hips levels. The intra-abdominal cavity being relatively spared from significant fat accumulation, they may avoid the complications associated with the metabolic syndrome. Consequently, indirect measures of central fat distribution such as the waist circumference may be better markers of obesity-related comorbidities such as coronary artery disease [9]. Also, abdominal obesity may be of importance in the postoperative period. Not surprisingly, in a large cohort of patients who underwent isolated CABG, a strong association between BMI-defined obesity and adverse postoperative events was found. More interestingly, adiposity, assessed according to WC, was associated with an increased risk of postoperative adverse events, independently of BMI. This data suggest that WC and BMI, both markers of different features of adiposity, are both associated with postoperative complications [10].
12.3.2 Cardiomyopathy
Cardiomyopathy is a common finding among the severely obese patients, and it can lead to diastolic and/or systolic heart failure [11]. A significant accumulation of body fat causes an increase in overall metabolism, blood volume, and cardiac output. The heart chambers dilate in response to a chronic volume overload, thus increasing stress on ventricular walls. The myocardium adapts to this phenomenon through hypertrophy. This eventually leads to left ventricular dysfunction and diastolic heart failure. With long-standing severe obesity, myocardial hypertrophy fails to compensate for this additional stress imposed on the ventricles. Systolic heart failure may eventually develop. This often occurs after several years of severe obesity (BMI > 50 kg/m2) [12]. It should be noted that the ventricular remodeling associated with obesity may be reversible after significant weight loss [13–15]. Because pulmonary comorbidities are frequently found in this population, right ventricular dysfunction is a common finding. Repeated hypoxic episodes lead to pulmonary hypertension and ensuing right ventricular dilatation and eventually failure [14].
12.3.3 Respiratory Comorbidities
Obesity impacts respiratory function in various ways. The burden on respiratory mechanics and diaphragmatic excursion will be greater if the bulk of tissue is in the upper chest and abdominal area, as seen with central obesity. Chest compliance is diminished, and reduced lung volumes confirm the presence of a restrictive syndrome. Also, the increased circulating blood volume adds to this restrictive physiology by decreasing lung compliance. The functional residual capacity (FRC) is primarily affecting lung volume. This lower FRC, especially in the supine position, often leads to lung volumes being lower than the closing capacity, causing ventilation-perfusion mismatch and hypoxemia even during normal breathing [16]. This phenomenon is increased during general anesthesia. Overall, obese subjects present an increased work of breathing, and to compensate, they tend to adopt a breathing pattern with reduced tidal volumes and higher respiratory rates. Obesity-induced bronchial hyperactivity is frequent in this population, and it can be resistant to standard asthma treatment [17]. An abundance of soft tissue in the cervical region predisposes to upper airway obstruction. Therefore, obstructive sleep apnea (OSA) affects up to 40% of obese patients [18]. Patients with severe undiagnosed OSA are particularly at risk of respiratory depression during the postoperative period, especially with the administration of opiates. The patient suffering from OSA who is not adequately managed with a continuous positive airway pressure (CPAP) could then present episodes of significant desaturation, even a few days after the procedure [19]. Moreover, the treatment of moderate to severe OSA with a CPAP reduces the risk of cardiovascular complications in the long term and should be instituted when feasible [20].
The obesity hypoventilation syndrome (Pickwickian syndrome) is characterized by daytime hypercapnia related to central hypoventilation. The physiopathology of this condition remains imprecise. It is estimated that this syndrome affects about 11% of severely obese patients suffering from OSA [21]. These patients are particularly vulnerable to postoperative respiratory complications and often present with significant right ventricular dysfunction. Of importance, waist circumference (WC) has been shown to be associated with an increased risk of postoperative atrial fibrillation, prolonged mechanical ventilation and reintubation, renal failure, and new postoperative renal replacement therapy, blood stream infection, sternal wound infections, and intensive care unit and hospital length of stay independently of BMI. These associations were independent of BMI, a marker of total adiposity in contrast to WC, which represents a marker of central obesity. These findings suggest that, besides total adiposity per se, fat mass distribution also influences clinical outcomes after isolated CABG [10].
12.3.4 Other Comorbidities
Obese patients have a higher incidence of type 2 diabetes, chronic kidney disease, osteoarthritis, hiatal hernias and certain types of cancer. They may also develop nonalcoholic fatty liver disease, which can progress to cirrhosis. The obesity-related state of chronic inflammation and impaired fibrinolysis place patients with high BMI at risk for thromboembolic complications, especially in the perioperative period.
12.4 Preoperative Evaluation
Preoperative assessment of obese patients should focus on their airway as well as their cardiopulmonary status.
12.4.1 Cardiac Assessment
Some obese patients presenting for surgery are relatively healthy, and thus, the need for further cardiac evaluation should be based on the patient’s specific risk factors for cardiovascular disease and the risks associated with the surgery itself [14]. A patient’s ability to perform at least four metabolic equivalents (METs) should be reassuring that its cardiopulmonary status is adequate to undergo most low- and intermediate-risk surgeries without further testing [22]. Unfortunately, obese patients may have limited functional capacity because of weight-related issues. Also, dyspnea on exertion, non-anginal chest pain and lower limb edema are frequent complaints in this population.
Features of the metabolic syndrome should be actively sought, as it is frequently associated with coronary artery disease [7]. A preoperative electrocardiogram should be obtained if coronary artery disease is suspected based on history or risk factors. A new left bundle branch block can be a sign of occult CAD. An ECG showing right axis deviation or a right bundle branch block should raise suspicion of possible right ventricular dysfunction and pulmonary hypertension, and further investigation is appropriate [14].
An algorithm for the assessment of severely obese individuals undergoing noncardiac surgery has been published and may help in planning appropriate investigations in this population [14]. Known coexisting cardiac conditions should be stable prior to surgery and optimized if necessary.
12.4.2 Respiratory Assessment
OSA is usually diagnosed based on the apnea-hypopnea index (AHI) following a sleep study using an overnight polysomnography. Treatment with a CPAP is recommended for moderate (15–30 events/h) and severe OSA (>30 events/h) [23]. A polysomnography is a costly exam, time-consuming, and not always readily available in the perioperative setting. The “STOPBANG” questionnaire (Table 12.2) is validated in the surgical population for preoperative screening for OSA [24]. It has a high sensitivity in detecting severe OSA using a cutoff score ≥3 (100% sensitivity) but with only moderate specificity. In the obese population, using a cutoff score of four provides a better balance between sensitivity and specificity, yielding lower false-positive rates. If a patient scores 0–2 on the questionnaire, moderate or severe OSA can be confidently ruled out [25].
Table 12.2
“STOPBANG” questionnaire to detect obstructive sleep apnea (OSA)
Snoring | Snoring loudly |
Tiredness | Daytime fatigue |
Observed | Stop breathing observed during sleep |
Pressure | High blood pressure |
BMI | BMI ≥ 35 kg/m2 |
Age | ≥50 years |
Neck circumference | ≥40 cm |
Gender | Male |
OSA increases the risk of perioperative complications [23]. Identifying patients at high risk for OSA before surgery will target perioperative precautions and interventions that may help reduce perioperative complications. OSA should ideally be diagnosed during the preoperative period in order to adjust a continuous positive airway pressure (CPAP) device, and this may decrease perioperative complication rates. According to the Society of Anesthesia and Sleep Medicine guidelines, when management of comorbid conditions has been optimized, patients with diagnosed, partially treated, untreated, or suspected OSA may proceed to surgery, provided that strategies for mitigation of postoperative complications are implemented [23]. The obesity hypoventilation syndrome can be suspected in patients with severe OSA and severe obesity. Elevated serum bicarbonate levels (>27 mmol/L) should raise suspicion that this condition is present. The diagnosis can be confirmed during the preoperative assessment by arterial blood gases showing a PCO2 > 45 mmHg and a PO2 < 70 mmHg (at room air) if there are no other pathologies to explain these findings [25].
12.4.3 Airway Assessment
12.4.3.1 Predictive Factors of Difficult Mask Ventilation and Intubation
Until recently, the literature reported risk factors for difficult mask ventilation (DMV) and difficult intubation (DI) separately. Kheterpall et al. have published a retrospective series of over 175,000 anesthesias [26]. In this cohort, he reported an incidence of 0.04% (1:250) of the combination of DMV as DI. He also established a list of predictors (Table 12.3) regrouped in risk index classification (Table 12.4). According to this new classification, it is rather the addition of factors that increases the risk of difficult ventilation/intubation and BMI is only one factor among many. But in an obese patient, it is frequent to find several concomitant factors that increase the risk of managing the airway: increased neck circumference, obstructive sleep apnea, limited cervical extension, male gender, and a BMI > 30 kg/m2. Thus, an obese patient increases quickly his/her risks classification of DMV and DI increased. The environment may also influence the level of the DI process in obese patient. A recent publication showed an increase from 8 to 16% of DI in obese patient in the ICU compared with a similar population in the OR [27]. During the preoperative evaluation, the possibility of an awake intubation should be discussed with the patient. Final decision regarding airway management should be taken by the anesthesiologist in charge on the day of the surgery following optimal positioning on the operating table.
Table 12.3
Difficult mask ventilation combined with difficult laryngoscopy prediction scorea
Predictor | Weighted points | Unweighted points |
|---|---|---|
Mallampati III or IV | 6 | 1 |
Neck radiation changes or neck mass | 5 | 1 |
Male sex | 5 | 1 |
Limited thyromental distance | 5 | 1 |
Presence of teeth | 5 | 1 |
Body mass index ≥30 kg/m2 | 4 | 1 |
Age ≥ 46 | 3 | 1 |
Presence of beard | 3 | 1 |
Thick neck | 2 | 1 |
Sleep apnea | 2 | 1 |
Unstable cervical spine or limited neck extension | 2 | 1 |
Limited or severely limited jaw protrusion | 2 | 1 |
Total possible | 44 | 12 |
Validation cohort c-statistic | 0.81 (0.78–0.84) | 0.81 (0.78–0.84) |
Table 12.4
Risk index classification system—validation cohorta
Preoperative risk class | Total patients, (n) | Patients with DMV combined with DL, % (n) | Odds ratio (IC 95%) |
|---|---|---|---|
Class I (0–3 risk factors) | 57,439 | 0.18 (107) | Reference |
Class II (4 risk factors) | 10,534 | 0.47 (50) | 2.56 (1.83–3.56) |
Class III (5 risk factors) | 5815 | 0.77 (45) | 4.18 (2.95–5.93) |
Class IV (6 risk factors) | 2775 | 1.69 (47) | 9.23 (6.54–13.04) |
Class V (7–12 risk factors) | 1509 | 3.31 (50) | 18.4 (13.1–25.8) |
12.4.3.2 Evaluation of the Risk of Aspiration
It was demonstrated recently that the obese patient’s stomach contains no more gastric liquid and that this liquid is not more acidic than in nonobese patients [28]. In addition, normal gastroesophageal physiology is preserved in this population [29]. Clinical data allow us to omit systematic rapid sequence induction and the cricoid pressure in obese patients. But the use of antacid gastric preparation such as histamine H2 receptor antagonist or protons pump inhibitors is simple to use and should be prescribed to all obese patients in order to get safe stomach content, i.e., gastric volume < 25 mL and pH < 2.5 to prevent pulmonary damage when aspiration may occur [30]. The most frequently described aspirations setting in obese patients were observed in the presence of difficulties during maneuvers of ventilation or intubation.
However, obese subjects who underwent bariatric surgery have altered function of the esophagogastric junction and are at risk of regurgitation as this is the case in nonobese patients presenting gastroesophageal reflux symptoms [31]. In addition to gastric preparation, these patients should be induced with a rapid sequence and cricoid pressure. The premedication should be mild. Sublingual or oral benzodiazepines give very good results.
12.5 Anesthesia Management
12.5.1 Airway Management
Whether for inducing general anesthesia or for an awake intubation, the adequate positioning of the obese patient and the optimal preoxygenation are essential for a safe airway management.
12.5.2 Position for Preoxygenation and Intubation
The supine position is undoubtedly the worst enemy regarding the oxygen reserve in the obese patient. The weight of the abdomen compresses the functional residual capacity (FRC) and even more when the patient has a paralyzed diaphragm. The impact on FRC of different positions of the obese patient on the operating table has been evaluated [32]. Preliminary results suggest that reverse Trendelenburg and beach chair positions are superior to the decubitus position to ensure a safe apnea period [32]. Furthermore, when a preoxygenation is performed in spontaneous ventilation, the patient is often unable to overcome the restriction of lung compression due to the abdominal content. Therefore, oxygen reserve is built up via deeper lung volumes. Using a CPAP or a BiPAP can defeat this restrictive syndrome and provide efficient preoxygenation [33].
We recently showed that reverse Trendelenburg position used in association with noninvasive positive pressure preoxygenation compared with the association of beach chair position with spontaneous ventilation allowed 16% longer (42 s) safe non-hypoxic apnea time (Sat O2 > 90%) (258 ± 42 vs. 217 s ± 17, p = 0.0053) and faster recovery to Sat O2 = 97% (68 ± 11 vs. 88 s ± 17, p = 0.029) following ventilation when reaching Sat O2 = 90% after tracheal intubation [34]. These results confirmed our laboratory data which demonstrated in awake obese patient the advantage of the association of reverse Trendelenburg position and noninvasive positive pressure preoxygenation to get better FRC compared to the association of beach chair or supine position with spontaneous ventilation [35] (Fig. 12.3).