The Renal System and Anesthesia for Urologic Surgery
The kidneys play a central role in implementing and controlling a variety of homeostatic functions, including excreting metabolic waste products in the urine while keeping extracellular fluid (ECF) volume and composition constant (Stafford-Smith M, Shaw A, Sandler A, Kuhn C. The renal system and anesthesia for urologic surgery. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013:1400–1439). Renal dysfunction may occur as a direct result of surgical or medical disease, prolonged reduction in renal oxygen delivery, nephrotoxin insult, or a combination of the three.
I. Renal Anatomy and Physiology
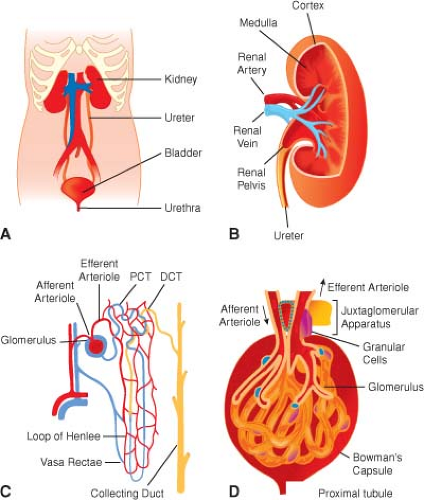
Gross Anatomy (Fig. 49-1A)
Renal pain sensation is conveyed back to spinal cord segments T10 to L1 by sympathetic fibers. Sympathetic innervation is supplied by preganglionic fibers from T8 to L1. The vagus nerve provides parasympathetic innervation to the kidney, and the S2 to S4 spinal segments supply the ureters.
The bladder is located in the retropubic space and receives its innervation from sympathetic nerves originating from T11 to L2, which conduct pain, touch, and temperature sensations. Bladder stretch sensation is transmitted via parasympathetic fibers from segments S2 to S4. Parasympathetics also provide the bladder with most of its motor innervation.
The prostate, penile urethra, and penis also receive sympathetic and parasympathetic fibers from the T11 to L2 and S2 to S4 segments. The pudendal nerve provides pain sensation to the penis via the dorsal nerve of the penis.
Ultrastructure (Fig. 49-1B–D)
The parenchyma of each kidney contains approximately 1 × 106 tightly packed nephrons (structural units of the kidneys), each one consisting of a tuft of capillaries
(glomerulus) invaginated into the blind, expanded end (glomerular corpuscle) of a long tubule that leaves the renal corpuscle to form the proximal convoluted tubule in the cortex.
The distal convoluted tubule comes into very close contact with the afferent glomerular arteriole, and the
cells of each are modified to form the juxtaglomerular apparatus, a complex physiological feedback control mechanism contributing in part to the precise control of intra- and extrarenal hemodynamics that is a hallmark feature of normally functioning kidneys.
Correlation of Structure and Function
Renal tissue makes up only 0.4% of body weight but receives 25% of cardiac output, making the kidneys the most highly perfused major organs in the body, and this facilitates plasma filtration at rates as high as 125 to 140 mL/min in adults.
Kidneys fulfill their dual roles of waste excretion and body fluid management by filtering large amounts of fluid and solutes from the blood and secreting waste products into the tubular fluid.
Glomerular Filtration
Production of urine begins with water and solute filtration from plasma flowing into the glomerulus via the afferent arteriole. The glomerular filtration rate (GFR) is a measure of glomerular function expressed as milliliters of plasma filtered per minute and is heavily influenced by arteriolar tone at points upstream (afferent) and downstream (efferent) from the glomerulus.
An increase in afferent arteriolar tone, as occurs with intense sympathetic or angiotensin II stimulation, causes filtration pressure and GFR to decrease.
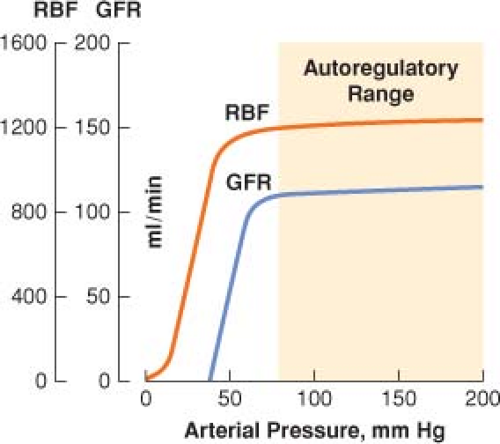
Autoregulation of Renal Blood Flow and Glomerular Filtration Rate
Renal blood flow (RBF) autoregulation maintains relatively constant rates of RBF and glomerular filtration over a wide range of arterial blood pressure (Fig. 49-2).
Autoregulation of urine flow does not occur, and above a mean arterial pressure (MAP) of 50 mm Hg, there is a linear relationship between and MAP and urine output.
Tubular Reabsorption of Sodium and Water
Active, energy-dependent reabsorption of sodium begins almost immediately as the glomerular filtrate enters the proximal tubule. (An adenosine triphosphatase pump drives the sodium into tubular cells, and chloride ions passively follow.)
At the loop of Henle in the collecting duct, water reabsorption is controlled entirely by antidiuretic hormone secreted by the pituitary gland.
The Renin–Angiotensin–Aldosterone System
Renin release by the afferent arteriole may be triggered by hypotension, increased tubular chloride concentration, or sympathetic stimulation.
Aldosterone stimulates the distal tubule and collecting duct to reabsorb sodium (and water), resulting in intravascular volume expansion.
Renal Vasodilator Mechanisms
Opposing the saline retention and vasoconstriction observed in stress states are the actions of atrial natriuretic peptide (ANP), nitric oxide, and the renal prostaglandin system. ANP is released by the cardiac atria in response to increased stretch under conditions of volume expansion.
Nitric oxide produced in the kidney opposes the renal vasoconstrictor effects of angiotensin II and the sympathetic nervous system, promotes sodium and water excretion, and participates in tubuloglomerular feedback.
II. Clinical Assessment of the Kidney
Measures such as urine output correlate only poorly with perioperative renal function, but much about the kidneys can be learned from knowing how effectively they clear circulating substances and from inspection of the urine (Table 49-1).
Table 49-1 Clinical Assessment of the Kidney | ||||||
|---|---|---|---|---|---|---|
|
III. Perioperative Nephrology
Pathophysiology. Altered renal function can be thought of as a clinical continuum ranging from normal compensatory changes seen during stress to frank renal failure.
The net result of modest activity of the stress response system is a shift of blood flow from the renal cortex to the medulla, avid sodium and water reabsorption, and decreased urine output.
A more intense stress response may induce a decrease in RBF and GFR by causing afferent arteriolar constriction. If this extreme situation is not reversed, ischemic damage to the kidney may result, and acute renal failure (ARF) may become clinically manifest.
Electrolyte Disorders (Table 49-2)
Table 49-2 Electrolyte Disorders
Hyponatremia (most common electrolyte disorder; symptoms are rare unless sodium values are <125 mmol/L)
Hypernatremia (sodium gain or water loss; serum sodium >145 mmol/L)
Disorders of potassium balance (skeletal muscle weakness, ileus, myocardial depression)
Hypocalcemia (laryngospasm)
Hypercalcemia (primary hyperparathyroidism, malignancy)
Hypomagnesemia (<1.6 mg/dL)
Hypermagnesemia (>4–6 mg/dL)
Table 49-3 Acid–Base Disorders
Metabolic acidosis (to determine the cause, the anion gap should be calculated)
Metabolic alkalosis (gastrointestinal acid loss)
Respiratory acidosis (acute and chronic causes can be differentiated by examining arterial pH, PaCO2, and HCO3− values)
Respiratory alkalosis (increased minute ventilation)
Mixed acid–base disorders (common in intensive care unit patients)
Acid–Base Disorders. Acid–base homeostasis involves tight regulation of HCO3− and PaCO2 (Table 49-3).
Acute Kidney Conditions
Acute kidney injury (AKI) is the preferred term for an acute deterioration in renal function. It is associated with a decline in glomerular filtration and results in an inability of the kidneys to excrete nitrogenous and other wastes. AKI frequently occurs in the setting of critical illness with multiple organ failure, and the mortality rate is alarmingly high (≤90%).
Prerenal azotemia is an increase in blood urea nitrogen associated with renal hypoperfusion or ischemia that has not yet caused renal parenchymal damage.
Intrinsic AKI includes injury caused by ischemia, nephrotoxins, and renal parenchymal diseases.
Postrenal AKI (Obstructive Uropathy). Downstream obstruction of the urinary collecting system is the least common pathway to established AKI, accounting for <5% of cases.
Nephrotoxins and Perioperative AKI (Table 49-4)
Chronic Kidney Disease (CKD). Patients with non–dialysis-dependent CKD are at increased risk of developing end-stage renal disease (ESRD). These patients have GFRs below 25% of normal. Patients with decreased renal reserve are often asymptomatic and frequently do not have elevated blood levels of creatinine or urea.
The uremic syndrome represents an extreme form of CRF, which occurs as the surviving nephron population and GFR decrease below 10% of normal. It results in an inability of the kidneys to perform their two major functions, regulation of the volume and composition of the ECF and excretion of waste products.
Table 49-4 Nephrotoxins Commonly Found in the Hospital Setting
Exogenous
Endogenous
Antibiotics (aminoglycosides, cephalosporins, amphotericin B, sulfonamide, tetracyclines, vancomycin)
Calcium (hypercalcemia)
Myoglobin (rhabdomyolysis)
Uric acid (hyperuricemia and hyperuricemia)
Anesthetic agents (methoxyflurane, enflurane)
Hemoglobin (hemolysis)
NSAIDs (aspirin, ibuprofen, naproxen, indomethacin, ketorolac)
Bilirubin (obstructive jaundice)
Chemotherapeutic–immunosuppressive agents (cisplatinum, cyclosporin A, methotrexate, mitomycin, nitrosoureas, tacrolimus)
Oxalate crystals
Contrast media
Paraproteins
NSAID = nonsteroidal anti-inflammatory drug.
Water balance in ESRD becomes difficult to manage because the number of functioning nephrons is too small either to concentrate or to fully dilute the urine. This results in failure both to conserve water and to excrete excess water.
Patients with uremic syndrome often require frequent or continuous dialysis.
Life-threatening hyperkalemia may occur in patients with CKD because of slower-than-normal potassium clearance (Table 49-5). Derangements in calcium, magnesium, and phosphorus metabolism are also commonly seen in patients with CKD.
Metabolic acidosis occurs in two forms in ESRD (hyperchloremic, normal anion gap acidosis and a high anion gap acidosis caused by an inability to excrete titratable acids).
Complications of the Uremic Syndrome (Table 49-6)
Drug Prescribing in Renal Failure. Clearance of most medications involves a complex combination of both hepatic and renal function, and drug level measurement or algorithms for specific drugs are often recommended.
Anesthetic Agents in Renal Failure. With the possible exception of enflurane, anesthetic agents do not directly
cause renal dysfunction or interfere with the normal compensatory mechanisms activated by the stress response.
Table 49-5 Factors Contributing to Hyperkalemia in Chronic Renal Failure
Potassium Intake
Increased dietary intake
Exogenous IV supplementation
Potassium salts of drugs
Sodium substitutes
Blood transfusion
GI hemorrhage
Potassium Release from Intracellular Stores
Increased catabolism or sepsis
Metabolic acidosis
β-Adrenergic blocking drugs
Digitalis intoxication
Insulin deficiency
SCh
Potassium Excretion
Acute decrease in GFR
Constipation
Potassium-sparing diuretics
ACE inhibitors (decreased aldosterone secretion)
Heparin (decreased aldosterone effect)
ACE = angiotensin-converting enzyme; GFR = glomerular filtration rate; GI = gastrointestinal; IV = intravenous; SCh = succinylcholine.
Table 49-6 The Uremic Syndrome
Water Homeostasis
ECF expansion
Electrolyte and Acid–Base
Hyponatremia
Hyperkalemia
Hypercalcemia or hypocalcemia
Hyperphosphatemia
Hypermagnesemia
Metabolic acidosis
Cardiovascular
Heart failure
Hypertension
Pericarditis
Myocardial dysfunction
Dysrhythmias
Respiratory
Pulmonary edema
Central hyperventilation
Hematologic
Anemia
Platelet hemostatic defect
Immunologic
Cell-mediated and humoral immunity defects
Gastrointestinal
Delayed gastric emptying, anorexia, nausea, vomiting, hiccups, upper GI tract inflammation or hemorrhage
Neuromuscular
Encephalopathy, seizures, tremors, myoclonus
Sensory and motor polyneuropathy
Autonomic dysfunction, decreased baroreceptor responsiveness, dialysis-
associated hypotension
Endocrine-Metabolism
Renal osteodystrophy
Glucose intolerance
Hypertriglyceridemia
ECF = extracellular fluid; GI = gastrointestinal.
If the chosen anesthetic technique causes a protracted reduction in cardiac output or sustained hypotension that coincides with a period of intense renal vasoconstriction, renal dysfunction or failure may result.
Significant renal impairment may affect the disposition, metabolism, and excretion of commonly used anesthetic agents (with the exception of the inhalational anesthetics).
Induction Agents and Sedatives
Ketamine is less extensively protein bound than thiopental, and renal failure appears to have less influence on its free fraction.
Propofol undergoes extensive, rapid hepatic biotransformation to inactive metabolites that are renally excreted.
AKI appears to slow the plasma clearance of midazolam.
Opioids
Chronic morphine administration results in accumulation of its 6-glucuronide metabolite, which has potent analgesic and sedative effects.
Meperidine is remarkable for its neurotoxic, renally excreted metabolite (normeperidine) and is not recommended for use in patients with poor renal function.
Hydromorphone is metabolized to hydromorphone-3-glucuronide, which is excreted by the kidneys. This active metabolite accumulates in patients with renal failure and may cause cognitive dysfunction and myoclonus.
Codeine has the potential for causing prolonged narcosis in patients with renal failure and is not recommended for long-term use.
Fentanyl appears to be an acceptable choice in patients with ESRD because of its lack of active metabolites, unchanged free fraction, and short redistribution phase. Small to moderate doses, titrated to effect, are well tolerated by uremic patients.
Remifentanil is rapidly metabolized by blood and tissue esterases, and renal failure has no effect on the clearance of remifentanil.
Muscle relaxants are the most likely group of drugs used in anesthetic practice to produce prolonged effects in
ESRD because of their dependence on renal excretion (Table 49-7).
Table 49-7 Nondepolarizing Muscle Relaxants in Renal Failure
Drug
% Renal Excretion
Half-Life (hr) Normal/ESRD
Renally Excreted Active Metabolite
Use in ESRD
Pancuronium
30
2.3/4–8
+
Avoid
Pipecuronium
37
1.8–2.3/4.4
+
Avoid
Doxacurium
30
1.7/3.7
−
Avoid
Vecuronium
30
0.9/1.4
+
Avoid infusion
Rocuronium
30
1.2–1.6/1.6–1.7
−
Variable duration
Atracurium/cis-atracurium
<5
0.3/0.4
−
Normal
ESRD = end-stage renal disease.
Provided the serum potassium concentration is not dangerously elevated, succinylcholine (SCh) use can be justified as part of a rapid sequence induction technique because its duration of action in patients with ESRD is not significantly prolonged.
Concern about the increase in serum potassium levels after SCh administration (0.5 mEq/L in normal subjects) implies that the serum potassium level should be normalized to the extent possible in patients with renal failure, but clinical experience has shown that the acute, small increase in potassium after administration of SCh is generally well tolerated in patients with chronically elevated serum potassium levels.
Anticholinesterase and Anticholinergic Drugs
Full access? Get Clinical Tree