The Premature and Low–Birth-Weight Infant
David M. De Iulio MD, FAAP
Steven J. Kovacs MD, FAAP
Wendy McKenney MS, ARNP
INTRODUCTION
Since the advent of neonatology in the early 1960s, the management of premature and low–birth-weight newborns has undergone dramatic changes. Ever-smaller babies are surviving and as cost-containment efforts, which mandate the earliest possible discharges from neonatal intensive care units, (NICUs), increasing numbers of preterm babies are discharged home with unresolved health care needs and complex care issues. Graduates of NICUs experience a much higher rate of hospital readmissions during their first year than do their healthy full-term counterparts. This chapter reviews common and practical concerns for providers dealing with these infants before, during, and after NICU discharge. It is a basic guide to understanding the needs of such infants.
COMMON CONCERNS FOR PRETERM AND LOW–BIRTH-WEIGHT INFANTS
Primary care of preterm infants begins long before hospital discharge. The designated local primary care provider should communicate frequently with NICU staff from delivery to discharge to ensure that the provider is fully aware of the infant’s clinical course and has a broad perspective of both baby and family. A discharge planning meeting may be necessary for some infants who have special needs (eg, oxygen therapy or monitoring equipment). The provider’s participation in such a meeting is ideal but may be impractical. The American Academy of Pediatrics (AAP) (1998a) recently has published guidelines for predischarge collaboration between hospital staff and post-discharge provider, which should include arrangements for the following:
Home nursing visits
Any medical equipment going home with the baby
Community referrals
Specialist appointments, if indicated
Plans for developmental follow-up
Hearing and vision screening
Immunization status
Diet and medications
Parental caregiving
Education needs
Questionable home environment or family issues
The NICU care provider should create a detailed discharge summary that includes ongoing problems and risk factors with recommendations for management.
Providers who do not receive a discharge summary should contact the NICU to obtain one. It should contain recommendations specific to that infant’s needs and should be a part of the clinician’s office records.
The baby’s first visit to the primary care provider should occur within a few days after discharge. The discharging neonatologist or neonatal nurse practitioner will recommend the timing of this initial visit according to the baby’s specific needs. Some infants may need weekly or biweekly visits after discharge until adequate growth has been established, and the transition to the home environment is considered complete (Trachtenbarg & Miller, 1995).
Nutrition
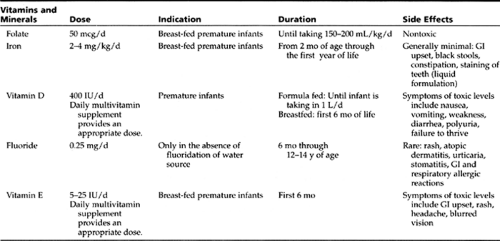
At birth, premature infants are at a nutritional disadvantage, because they are born without the nutrient stores that the fetus normally accrues during the last trimester (Pursley & Cloherty, 1998). The nutrition goal for preterm infants is to approximate the rate of intrauterine growth (Nutrition Committee, Canadian Pediatric Society, 1995). Smaller (less than 1800 g) and younger (less than 34 weeks) premature infants require formula specifically designed to provide higher protein, mineral, and caloric contents. Human breast milk requires supplementation with special fortifiers to provide for those needs. Table 16-1 provides information about the vitamin and mineral supplementation needs of premature infants.
• Clinical Pearl
Poly-Vi-Sol with iron preparation contains sufficient amounts of vitamins A, D, C, E, B1, B2, B6, B12, niacin, and iron. It generally is given as 1 mL (1 dropperful) every 24 hours.
At discharge, preterm infants must be able to demonstrate competency with breastfeeding or bottle feeding. Providers can discontinue nutrient supplementation at a corrected age of 36 weeks in most infants or when exclusive breast-feeding has been established.
• Clinical Pearl
The primary care provider should follow infant and mother closely to ensure the baby’s adequate growth and hydration. Obtaining a 24-hour diet history at the first office visit helps determine adequacy of feeding. The provider also should review stooling and voiding patterns to ensure that they are appropriate for the baby’s chronologic age. The clinician should reinforce normal and expected patterns of feeding, including frequency of nursing. If the mother reports difficulty, she should feed the infant in the office so that the provider can assess the problem’s origin. A referral to a lactation specialist may help the mother–infant dyad improve breast-feeding skills (Trachtenbarg & Golemon, 1998).
The AAP recommends exclusive formula feeding or breastfeedings for the first 5 to 6 months of life (Lucas, 1993), with caregivers postponing the introduction of solid foods until this time. Because this recommendation is based on the maturational status of the infant’s nervous system, intestinal tract, and kidneys, premature infants need not necessarily wait until 5 to 6 months’ corrected age. Some babies are indeed ready at 5 to 6 months’ actual age, while others require more time. The primary care provider should be able to assess an infant’s developmental progress and make an appropriate recommendation.
Breast-fed Infants
Many experts believe that human milk is the preferred diet for all infants. Breast milk benefits premature infants in many ways. Milk secreted the first 4 to 6 weeks after premature delivery differs in composition from milk secreted after term. Though the fat contents are similar in both, the fat in preterm milk is of a smaller globule size, enhancing its absorption. Absorption of fat from human milk is 95% compared with 85% in preterm formula. Many of the long-chain fatty acids necessary for brain growth also are higher in human preterm milk (Harnosh, 1994). Before 32 weeks’ gestation, active transport of immunoglobulin G across the placenta does not begin in earnest and the immune system is immature. Thus, infants born before this time are immunologically disadvantaged (Goldman et al., 1994). The immunoglobulins present in human milk, however, offer protection to these vulnerable preterm babies. Studies have demonstrated fewer infections in breast-fed preterm infants (El-Mohandes et al., 1997; Goldman et al., 1994; Hylander, Strobino, & Dhanireddy, 1998). Moreover, all breastfed infants appear to have higher developmental and IQ scores (Tudehope & Steer, 1996; Horwood & Fergusson, 1998; Lucas, 1993) than do formula-fed babies.
Formula-Fed Infants
Preterm infants whose mothers do not plan to breast-feed require a formula designed to provide increased calories, protein, and minerals. These infants generally are switched to a term formula at 36 weeks’ corrected age. Wheeler and Hall (1996) concluded that infants who weighed less than 1800 g at birth and were kept on a preterm formula for 8 weeks after discharge were longer and had larger head circumferences 12 weeks after discharge than did preterm infants who received term formula. The short-term advantages are clear, but long-term benefits are less certain. Extremely premature (less than 28 weeks) babies often experience delays in reestablishing adequate growth after birth. Therefore, recommendations are to discharge these infants home for several months on an intermediate formula, such as Neocare, which has 22 kcal/oz. The long-term benefits of this approach have not been documented clearly.
Occasionally, an infant with high metabolic expenditures (eg, chronic lung disease, malabsorption, neurologic impairment, congenital heart disease) requires a higher kcal/oz formula after discharge as well. These babies are at slightly higher risk for hyperosmolar dehydration and may need monitoring of their electrolyte levels if they experience vomiting or diarrhea (Trachtenbarg & Golemon, 1998).
Growth
Adequate growth of premature babies should be documented using charts designed specifically for them, available through the AAP. Preterm infants experience catch-up growth that exceeds the rate of growth of term babies. Maximal catch-up growth occurs between 36 and 44 weeks’ chronologic age. Babies who are small for gestational age experience the most rapid growth in the first 3 months of life, with catch-up growth continuing to school-age. A small percentage of infants never catch up to the term infant (Trachtenberg & Golemon, 1998a; Dusick, 1997).
Poor growth and nutrition have major effects on developmental outcomes (Dusick, 1997). Providers must evaluate infants with poor growth for swallowing difficulties, gastroesophageal reflux, anemia, hypoxemia, or other factors (eg, feeding behaviors) that may be
compromising ability to obtain sufficient nutrition. Providers can then initiate appropriate therapeutic interventions.
compromising ability to obtain sufficient nutrition. Providers can then initiate appropriate therapeutic interventions.
Immunizations
The AAP releases a standard, up-to-date pediatric immunization schedule each year, which is used for all babies, including premature infants. No schedules are specific for premature infants, who should receive all childhood vaccines at the correct chronologic age, not at corrected gestational age. Currently, the only exception is for the first hepatitis B vaccine, which should be administered when the baby weighs 2 kg if the mother is hepatitis B surface antigen-negative. Studies have demonstrated higher seroconversion in the group vaccinated at a weight greater than 2 kg (AAP, 1997). Refer to Chapter 13, Immunizations, for information concerning specific preparations and the timing of their administration.
Infants with evolving neurologic disorders should not receive pertussis vaccine. They should receive pediatric Diptheria and Tetanus vaccine (without the pertussis component present in the DPT vaccine) instead.
Sleep
Prone sleeping has been associated with a higher incidence of sudden infant death syndrome (SIDS). In 1992, the AAP began recommending a supine or side-lying sleeping position for healthy term infants. Since then, the United States has experienced a 15% to 20% decrease in the incidence of SIDS (AAP, 1996). The risks and benefits of supine sleeping must be weighed individually for other than healthy term infants. If no contraindications to supine or side sleeping exist, the infant is switched to the supine position before discharge. Infants with neuromuscular disorders may need positioning that prevents secretions from pooling in the back of the throat. Babies with craniofacial abnormalities or evidence of upper airway obstruction require a prone sleeping position.
To prevent smothering, parents must avoid using soft bedding and loose covers, including baby quilts. They must evaluate use of crib bumpers carefully, ensuring that the infant’s head will not become lodged between bumper pad and crib rail (AAP, 1996).
In addition to sleep positioning, parents must be aware that premature infants sleep more hours total than term infants but experience more frequent awakenings. Transitioning the baby from the brightly lit and noisy nursery to the quieter home environment may take some time. Some parents have found that soft music or lighting helps soothe the baby during this time (Dusick, 1997).
COMMON CONDITIONS IN PREMATURE AND LOW–BIRTH-WEIGHT INFANTS
Cryptorchidism
Premature male infants are at risk for cryptorchidism (nondescended testes). Embryologically, the testes begin to descend from abdomen to scrotum between 28 to 30 weeks’ gestation. By term, 95% of testes in infants are in the scrotum; most are fully descended by 6 to 9 months. At 1 year, testes remain undescended in only 0.7% of infants.
Cryptorchidism is associated with future development of testicular cancer. Surgical consultation will be necessary if the testes are not descended by 1 year, because it is unlikely for this condition to change after that time (Trachtenburg & Goleman, 1998; Kaplan, 1994).
Inguinal Hernias
Inguinal hernias are more common in premature males who have required prolonged mechanical ventilation. They may be repaired before discharge, because the risk for incarceration is high due to the small size of the inguinal ring. Infants with chronic lung disease may be discharged with plans for hernia repair when their respiratory status has improved. In any case, a hernia that is present at or discovered after discharge requires surgical intervention. These infants require close monitoring. Parents need to learn the signs of hernia incarceration and strangulation (Trachtenburg & Goleman, 1998; Ringer, 1998).
Bronchopulmonary Dysplasia
As technologic support for preterm infants has improved, the definition of bronchopulmonary dysplasia (BPD) has evolved (Bancalari et al., 1979; Northway, Rosan, & Porter, 1967). Shennan et al. (1988) define BPD as oxygen dependence at 36 weeks’ postconceptional age, with radiologic abnormalities and a history of assisted ventilation. Infants with BPD have an increased risk for respiratory morbidity in the first few years of life. Studies have suggested using the term chronic lung disease (CLD) to describe these babies (Hack et al., 1991). Diagnosis of BPD at 28 days is still useful, however, because some who subsequently improve and are asymptomatic at discharge (and thus do not have CLD) still have abnormal pulmonary function tests (Abbasi & Bhutani, 1990; Myers et al., 1986).
Infants with BPD are at greater risk of rehospitalization with concurrent respiratory infections. They have less pulmonary reserve than do babies without BPD.
Epidemiology
Incidence of BPD and CLD is inversely proportional to birth weight and gestational age. It is directly proportional to the presence and severity of respiratory distress syndrome (RDS), the absence of antenatal corticosteroid exposure, aggressive mechanical ventilation, high oxygen requirements, pneumothoraces, fluid overload, hemodynamically apparent patent ductus arteriosus (PDA), sepsis, Caucasian race, male sex, and low Apgar score. In addition, heredity seems to play a role, because incidence is associated with a strong family history of asthma and an HLA-A2 haplotype (Farrell & Fiascone, 1997; Abman & Groothius, 1994; Nickerson & Taussig, 1980).
Several advances in neonatal care responsible for the decreasing incidence of CLD may be responsible for the continued high incidence of BPD. These advances include the following:
Maternal antenatal steroids. These decrease both the incidence of RDS and the need for mechanical ventilation and high oxygen supplementation. They also increase the concentration of antioxidants in premature lungs; these mitigate the toxic effects of supplemental oxygen.
Surfactant therapy. This decreases mortality but has no real effect on incidence of BPD. This is probably because of the increasing survival of extremely premature babies, whose pulmonary anatomic immaturity complicates
their course and their response to surfactant, and increases the time they spend on a ventilator exposed to supplemental oxygen.
Etiology
The cause of BPD is multifactorial. Both ventilator-associated barotrauma and exposure to high oxygen concentrations are essential to survival in the treatment of RDS, to which premature babies are frequently exposed. Ventilator-associated barotrauma and exposure to high oxygen concentrations, however, are capable of initiating an inflammatory reaction in the lungs (Farrell & Fiascone, 1997). Each of these factors causes an influx of neutrophils into the lungs, damaging them by generating oxygen-free radicals that release inflammatory mediators. These mediators, in turn, attract more neutrophils, creating an ongoing cycle of damage (Frank, 1992; Groneck et al., 1994). Premature babies have underdeveloped pulmonary antioxidant systems, making them particularly susceptible to oxygen toxicity.
Infection and colonization of the airway with Ureaplasma urealyticum has been identified as a risk factor for BPD. Pneumonia of any viral or bacterial etiology also may contribute to the development and severity of BPD, because pneumonia leads to activation of the inflammatory cascade, prolonging the need for mechanical ventilation.
Management
Different neonatal units use different modalities, including the following:
High-frequency ventilators
Synchronized ventilation (reduces the incidence of pneumothoraces, thus decreasing incidence of BPD)
Permissive hypercapnia (permits higher pCO2 values [less barotrauma])
Relative fluid restriction
Postnatal steroid administration (equivocal effects on the incidence of BPD because of wide variation in its use [dosage, timing, length of treatment] as well as confounding variables, such as the diverse approaches to ventilator strategies, fluid management, between centers)
Primary care providers deal with a spectrum of patients with BPD after they graduate from the NICU. Their conditions range from apparently asymptomatic with normal growth, to mild CLD requiring diuretics and enhanced nutritional needs, to more severe CLD requiring supplemental oxygen and various medications.
• Clinical Pearl
Outpatient management for patients with BPD revolves around the use of supplemental oxygen, pharmacologic treatment (ie, inhaled bronchodilators, and diuretics, with occasional use of corticosteroids), and appropriate nutrition. In addition to well child care, the main focus should be on growth, monitoring respiratory symptoms, and appropriate use of and weaning from medications. The primary care provider can perform these functions in conjunction with a pediatric pulmonologist in more severe cases.
Assuming the provider participated in the baby’s care prior to discharge, the initial outpatient visit should occur no longer than 2 weeks and ideally a few days after discharge. Follow-up visits may be necessary every few days to every few weeks until the infant has adjusted fully to home care and is doing well clinically. The provider periodically should attempt to reduce or eliminate therapies when the patient is asymptomatic and growing well.
Short-term oxygen saturation analysis in the office is not very predictive of long-term status and will fail to identify hypoxic episodes associated with stress, including sleeping, feeding, and crying. Episodic periods of desaturations can be measured and are not necessarily associated with apnea, bradycardia, or even cyanosis. They can therefore go undetected (Farrell & Fiascone, 1997; Garg et al., 1988).
As these patients grow, new lung tissue forms over a period of about 8 years, and pulmonary function improves. They may have abnormal pulmonary function for the rest of their lives, even if only noted in formal pulmonary function tests (Northway et al., 1990; Galdes-Sebaldt et al., 1989). They have experienced the double-edged sword of mechanical ventilation and supplemental oxygen during a critical period of lung development, ultimately leaving them with decreased numbers of larger-than-normal alveoli, gas exchange surface area, and pulmonary reserves.
Oxygen Therapy
Babies with BPD seem to require small amounts of oxygen for prolonged periods. Providers must avoid marginal oxygenation or hypoxia. The increased pulmonary vascular resistance that accompanies BPD improves with high-normal pO2 levels and is worsened by low-normal levels or hypoxia. General recommendations are to maintain oxygen saturations above 92% (Rush & Hazinski, 1992; Koops, Abman, Accurso, 1984). Low-flow oxygen therapy by nasal cannula is preferred, because high flow use is associated with drying of the nasal mucosa. Adequate growth is a marker of adequate oxygenation (Moyer-Mileur et al., 1996).
The weaning process should begin when the baby has experienced no respiratory symptoms for weeks and has shown an increase in growth of 20 to 40 g/d. Providers should record oxygen saturations in all states. If a baby does well in some states but not others, support must increase during those times of stress. Feeding and deep sleep are examples of times when oxygen requirements increase; perhaps they should serve as the last frontier in the weaning process. Good growth is a very good indicator of the child’s tolerance to the weaning process.
Hypoxia can occur during different states (eg, activity, feeding, rest, deep sleep) (Dusick, 1997). Monitoring at different times is essential to knowing the patient’s real oxygen requirements and to determining when weaning should begin. Oxygen therapy frequently is underused and stopped too quickly, resulting in alveolar hypoxia, poor growth, feeding difficulties, and pulmonary hypertension.
Pharmacologic Approaches
Airway hyper-reactivity frequently occurs in these patients, manifesting itself as increased work of breathing, inspiratory crackles, expiratory wheezing, and increased inability to handle concurrent viral illnesses. Bronchodilators are used widely for these problems and seem effective in decreasing the work of breathing, energy expenditure, and consequently caloric requirements. Of inhaled beta-mimetics, albuterol is most commonly used in NICUs.
Dosage starts at 1 mg in 2 to 3 mL of normal saline regardless of weight (0.2 mL of the 0.5% solution) and is adjusted either up or down, depending on its effects or the appearance of adverse reactions (eg, sustained tachycardia). Anticholinergic preparations like ipratropium bromide, especially in concert with beta-mimetics, also have been used successfully.
Dosage starts at 1 mg in 2 to 3 mL of normal saline regardless of weight (0.2 mL of the 0.5% solution) and is adjusted either up or down, depending on its effects or the appearance of adverse reactions (eg, sustained tachycardia). Anticholinergic preparations like ipratropium bromide, especially in concert with beta-mimetics, also have been used successfully.
One difficulty concerns the method of administration (eg, nebulizer, metered-dose inhaler) and the variable delivery to the distal airways. In addition, controversy exists over chronic versus as-needed use, based on asthma literature suggesting that chronic therapy ultimately increases mortality and worsens control (Barrington & Finer Neil, 1998). Methylxanthines (aminophylline and caffeine) also can be used as bronchodilators but are more commonly used in the acute management of apnea. Few babies are discharged home on methylxanthines for CLD. These medications have a narrow safety margin (more true for aminophylline than caffeine). Many outside factors affect serum levels, side effects are common, and frequent levels are necessary. Their role as a major treatment modality even in asthma has fallen dramatically.

Full access? Get Clinical Tree