1. The shaping of the skull base and contiguous structures is a dynamic process involving reciprocal influences between the cranial base, the pharynx, the face, and primary and secondary palates.
2. Multiple suture closure is often associated with premature closure of the base of skull sutures. These are called complex craniofacial synostosis and are often syndromic in nature.
3. Midfacial hypoplasia, malar maldevelopment, and hypoplastic sinuses may result in impaired secretion clearance and difficulty with mask ventilation.
4. Creation of a pharyngeal flap decreases the cross-sectional area of the airway to varying degrees. In some patients, this may change an adequate airway to a partially/totally obstructed one. These patients may exhibit hyperirritability or somnolence in the postoperative period. Return to the operating room (OR) to take down the flap may be lifesaving in this situation.
5. In its normal state, the tongue does not quite fill the entire oral cavity. Any disease state that alters the ratio of tongue mass to oral cavity volume can lead to problems with breathing and eating.
6. While not part of the immunologic system, the tongue base does have lymphoid tissue within it and is part of Waldeyer’s ring. An enlarged lingual tonsil may be the cause of an unanticipated difficult intubation.
7. For children with severe sleep apnea, carefully evaluate the need for further workup with chest x-ray, ECG, echocardiogram, and a pediatric cardiologist.
8. Noisy breathing is often an ominous sign in infants and small children. Purely inspiratory stridor usually indicates lesions in the upper part of the airway. Lesions distal to the vocal cords usually have primarily an expiratory stridor. Biphasic stridor is most characteristic of obstruction at the level of the subglottic space.
9. If at all possible, intubation should be avoided in croup patients. Infants less than 6 months old should be evaluated for an anatomic abnormality such as a subglottic hemangioma, which is more common than croup.
10. An x-ray study for stridor should never replace strong clinical suspicion and should never be attempted in a child in extreme distress.
11. Risks of pediatric tracheotomy include bleeding, infection, pneumothorax, pneumomediastinum, and subcutaneous emphysema. Pneumothorax is more of a risk in pediatric tracheotomy because of the more cranial position of the pleura in infants and small children.
12. Most thyroid disease will occur in the normal anatomic location; however, the embryology of the thyroid and its potential locations along the path of the thyroglossal duct, including the possibility of retrosternal thyroid disease, must prompt a thorough anatomic investigation prior to the surgical procedure.
13. Sufficient specialized airway equipment needs to be available for any anticipated difficult airway. The surgeon should be present for anesthetic induction; a tracheostomy set should be immediately available as well.
I. Development of the head and neck (1)
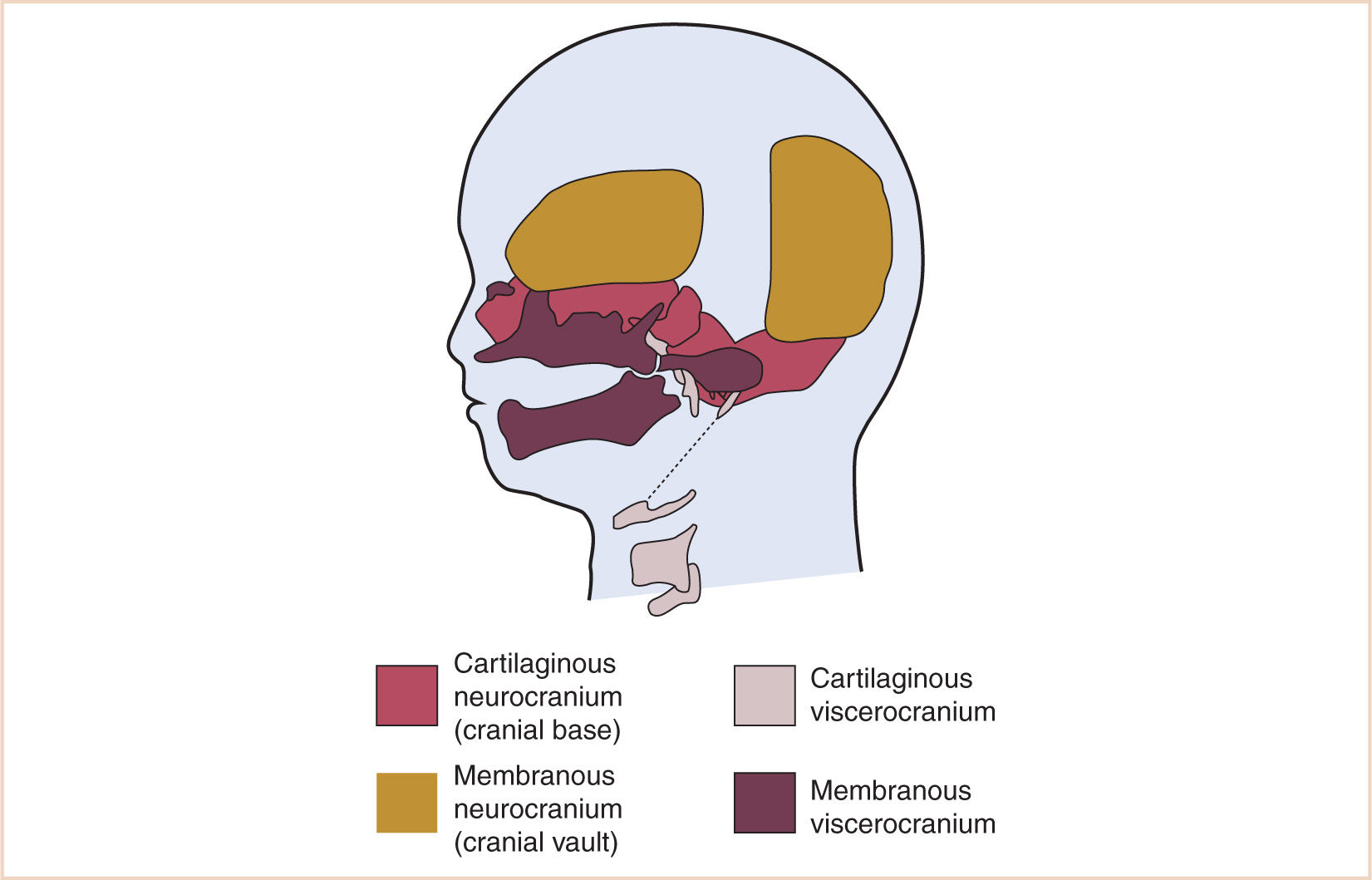
A. The skull develops from a membranous and cartilaginous neurocranium (Fig. 16.1).
1. The membranous neurocranium gives rise to the flat bones of the skull (the cranial vault), and the cartilaginous neurocranium (chondrocranium) forms the skull base.
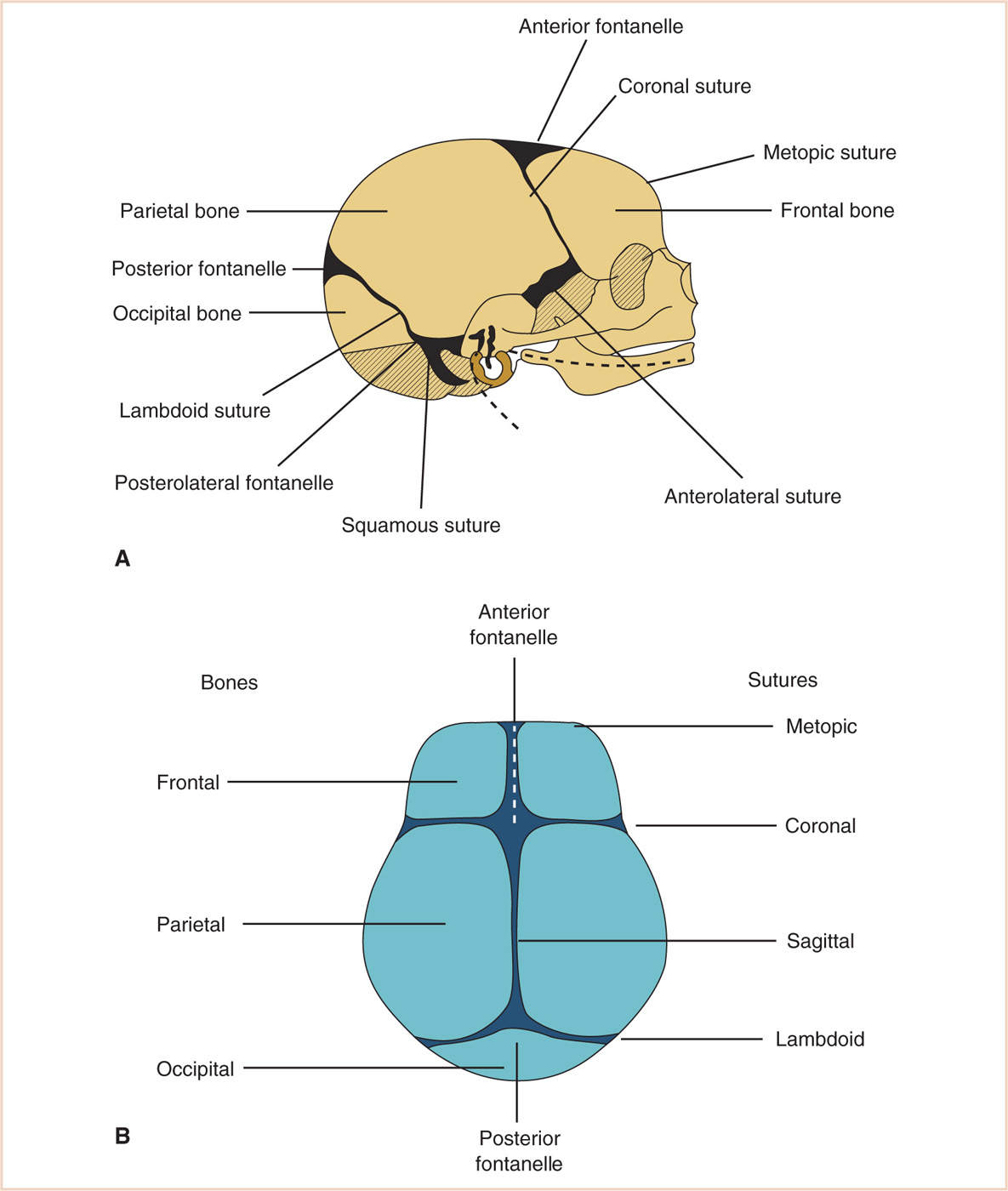
a. The flat bones of the neurocranium, which edge-to-edge form sutures, also form fontanelles where more than two bones meet. The frontal bones meet at the metopic suture, whereas the frontal and parietal bones meet at the coronal suture. The anterior fontanelle is the junction of the frontal and parietal bones. The sagittal suture is where the parietal bones meet. Posteriorly, the parietal bones meet the occipital bone at the lambdoid suture, and the posterior fontanelle is the junction of parietal and occipital bones (Fig. 16.2).
b. The base of the skull is formed from the cartilaginous neurocranium, which then becomes the base of the occipital bone, the sphenoid, ethmoid and petrous bone, and portions of the temporal bone. Where the base of the skull (primarily the squamous temporal bone) opposes the parietal bone, it becomes the squamous suture. Where the two bones meet the frontal and occipital bone, the anterolateral and posterolateral fontanelles are formed, respectively.
c. The cranial base, phylogenetically the oldest skeletal component, provides a floor for the calvarium and a roof for the face. The shaping of the skull base and contiguous structures is a dynamic process involving reciprocal influences between the cranial base, the pharynx, the face, and primary and secondary palates.
(1) During fetal life and early childhood, neural influences predominate because of the rapid growth of the brain.
(2) During postnatal development of the airway, nasal influences play a major role, and because of speech and nutritional requirements, the pharynx also influences the development of the skull base.
(3) The anterior portion of the skull base is the roof of the nasomaxillary complex, whereas the posterior portion of the cranial base is the roof of the nasopharynx.
(4) During development, the depth of the nasopharynx increases due to remodeling of the palate as well as changes in the angulation of the skull base, providing an enlarged nasal airway for the adult.
B. Craniovertebral development
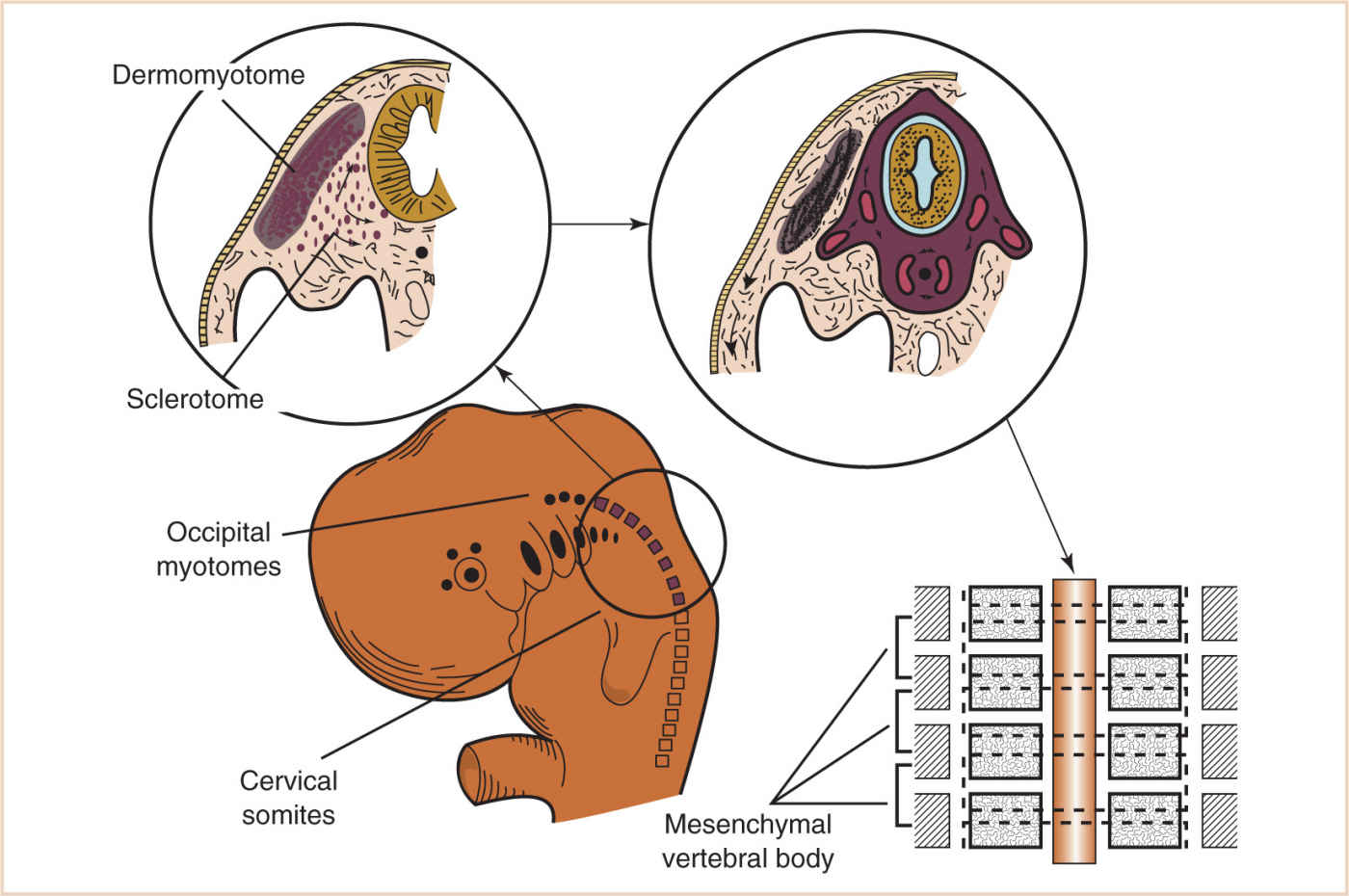
1. Embryonic mesoderm differentiates into three distinct regions early in development—paraxial, intermediate, and lateral mesoderm.
a. The paraxial mesoderm is a column of tissue on either side of the midline of the embryo, and at about the fourth week of development, it becomes divided into blocks of tissue called somites.
b. Whereas most of the muscles of the head are derived from mesenchyme of the branchial arches, three pairs of occipital myotomes migrate ventrally and cranially to form the muscles of the tongue.
c. Caudal to the occipital myotomes, each cervical somite differentiates into a ventromedial sclerotome and a dorsolateral dermomyotome (Fig. 16.3).
(1) The sclerotome consists of mesenchymal cells that migrate medially during the fourth week of development and surround the notochord.
(2) The caudal half of each sclerotome fuses with the cephalic half of the immediately succeeding sclerotome to form the mesenchymal vertebral body.
(3) Each vertebral body is therefore an intersegmental structure, with the notochord degenerating completely in the region of the vertebral body but enlarging in the intervertebral region to form the nucleus pulposus and intervertebral disc.
(4) The annulus fibrosus is derived from sclerotomic mesenchyme between adjacent vertebral bodies.
C. The face (Fig. 16.4)
1. Just as the neurocranium forms the cranial vault and base, the viscerocranium forms the face and is derived mainly from cartilage of the first two branchial arches.
a. Neural crest cells of the developing 3- to 4-week embryo, composed of ectoderm, are found at the junction of the neural plate and surface ectoderm. These neural crest cells then migrate to branchial arch mesoderm, and the face develops as a result of these massive cell migrations and their interactions with differentiating cells of mesodermal origin.
b. Those cells forming the frontonasal process are derived from the forebrain fold and migrate a relatively short distance as they pass into the nasal region.
c. Those cells that form the mesenchyme of the maxillary and mandibular processes have a considerably longer distance to migrate, as they must move into the branchial arches, where they surround the mesodermal muscle plates and contribute to the formation of the face.
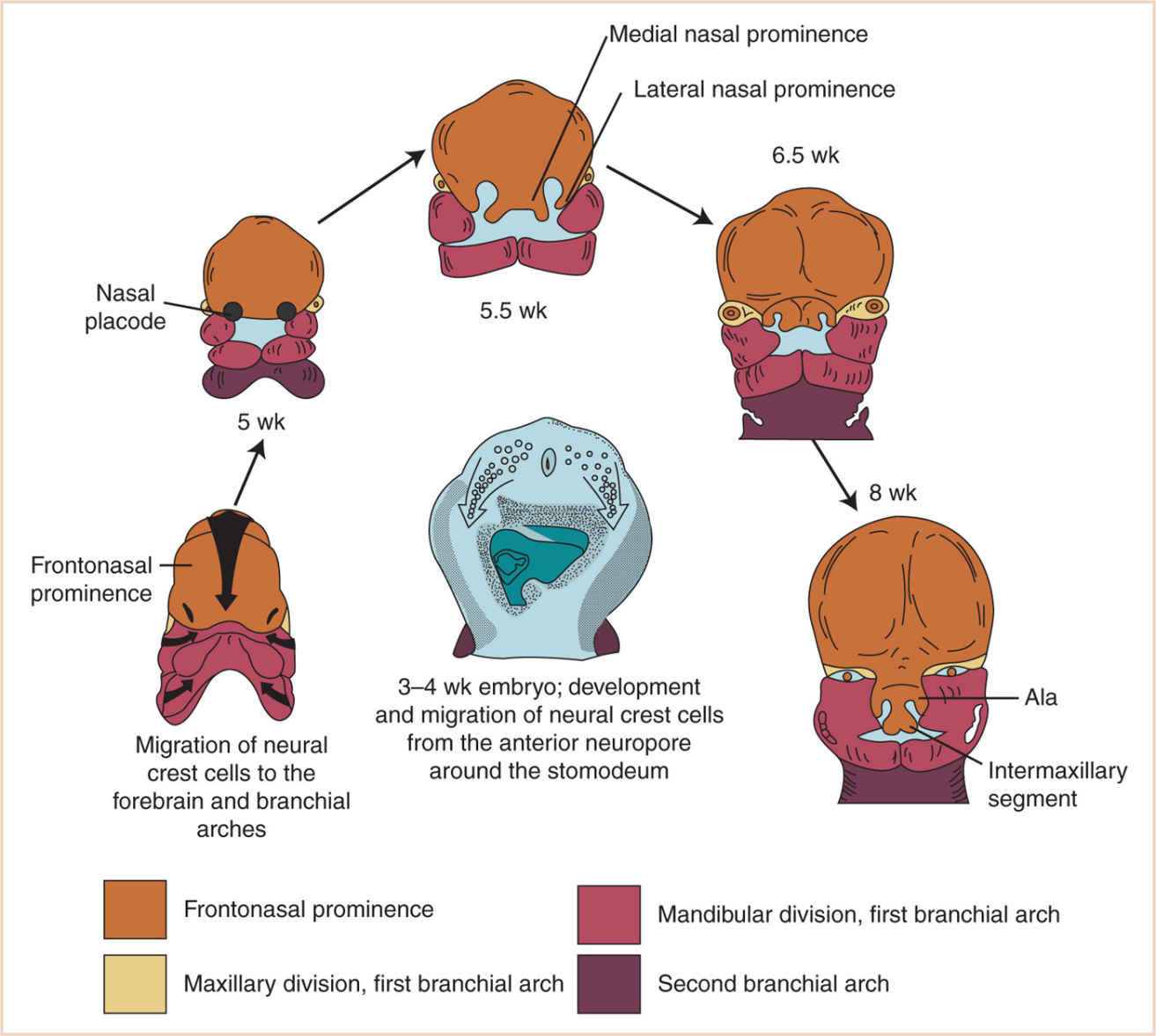
d. At 28 days, the face barely shows its eventual relationship to the five primordia from which it is derived:
(1) The frontonasal prominence, which is the cranial boundary of the primitive mouth (stomodeum)
(a) The frontonasal prominence develops in a craniocaudal fashion from mesoderm, under the influence of neural crest tissue.
(b) Ultimately, the keystone of the midface, the sides of the frontonasal prominence, gives rise to the nasal placodes.
(c) Small horseshoe-shaped ridges develop around the nasal placodes, called the medial and lateral nasal prominences, at about 33 days.
(2) The paired maxillary prominences (first branchial arch)
(3) The paired mandibular prominences (also first branchial arch). The mandibular prominences grow medially and begin to merge with each other by the end of the fourth week, forming the lower lip, the chin, and the mandible.
(4) Between the fifth and eighth weeks, the nasal pits form in the floor of the nasal placodes, and the maxillary prominences increase in size and grow medially.
(a) This moves the medial nasal prominences toward the median plane.
(b) The groove between the lateral nasal prominence and the maxillary prominence disappears during the merger, thus completing the “keystone.”
(5) The primitive mouth (stomodeum) appears as a slight depression in the surface ectoderm separated by the oropharyngeal membrane.
(a) This membrane ruptures at about 24 to 26 days’ gestation, and the primitive gut then communicates with the amniotic cavity.
(b) The involved germ layers are endoderm internally and ectoderm externally.
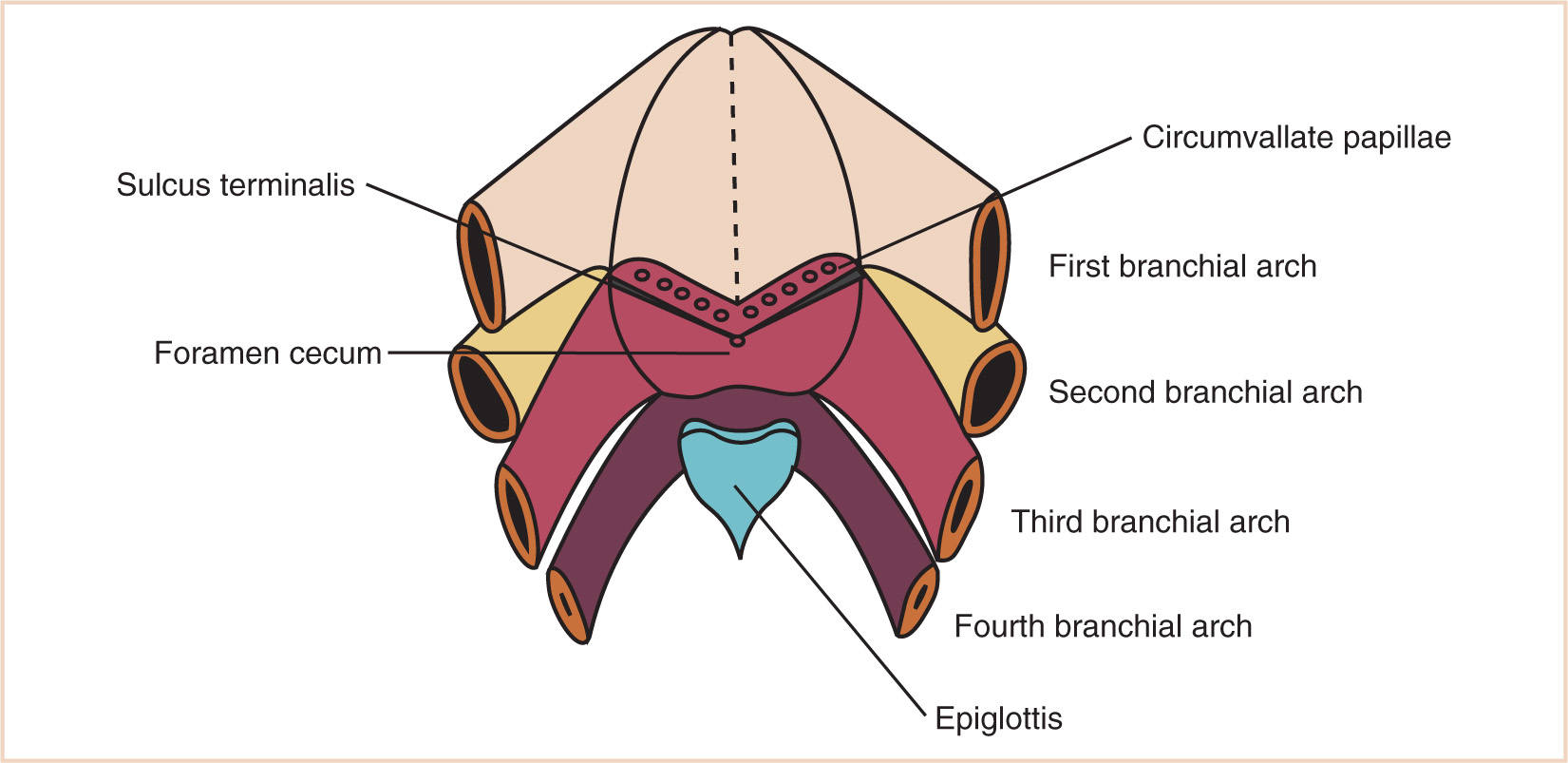
(6) The tongue surface arises primary from first arch mesenchyme, with significant contributions from third and fourth arches, hence its complex innervation by the facial nerve in the anterior two-thirds (that portion formed by the mandibular division of the first branchial arch) and the hypoglossal nerve (that portion formed by the third branchial arch) (Fig. 16.5).
(a) The foramen cecum is located posterior to the first branchial arch derivatives.
(b) The muscle bulk of the tongue arises primarily from occipital somites, explaining the hypoglossal nerve (XII) innervation of tongue musculature.
(i) The upper lip is formed by the merging of the maxillary prominences with the medial nasal prominences.
(ii) The lateral nasal prominences do not form part of the upper lip but rather form the alae of the nose.
(iii) The intermaxillary segment in the central portion of the upper lip area consists of three parts: the labial component, forming the philtrum, a maxillary component associated with the four incisor teeth, and a palatal component, which becomes the primary palate.
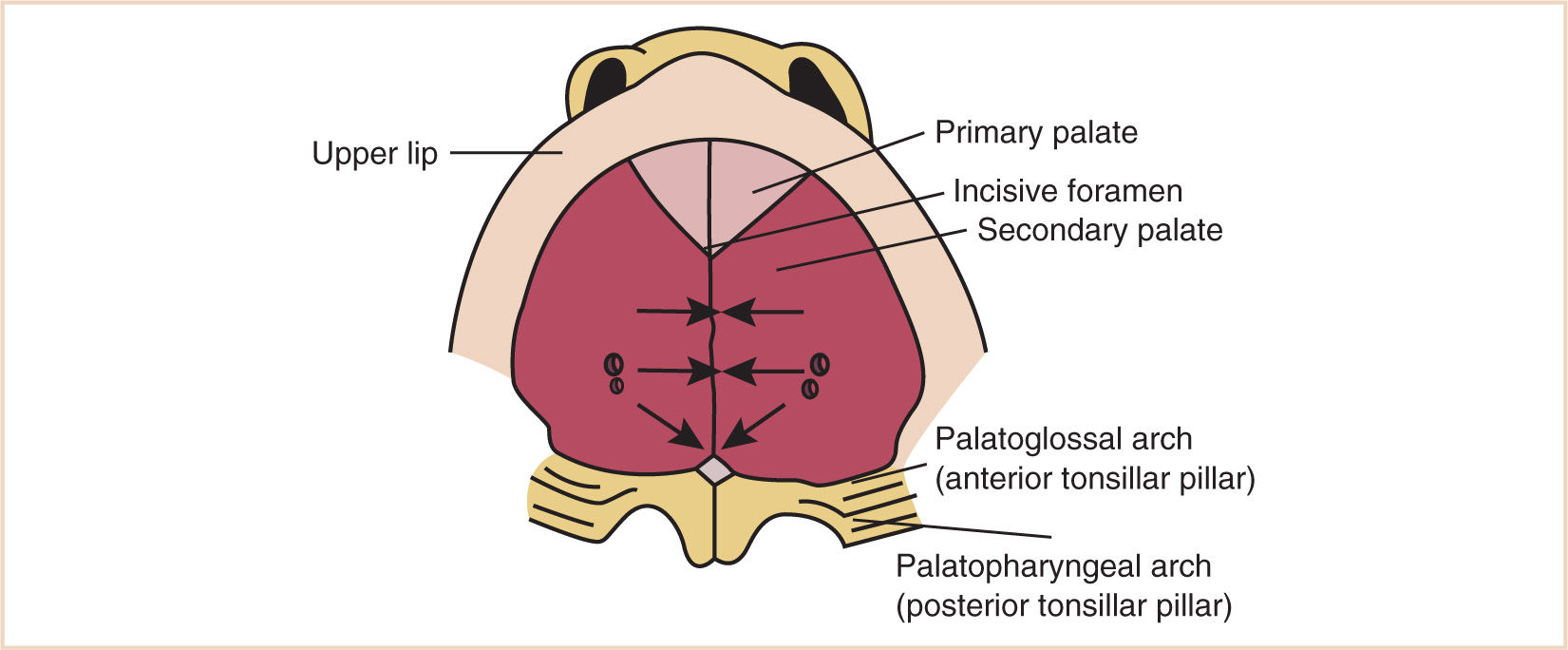
(c) The development of the palate (Fig. 16.6) serves to divide the nasomaxillary complex from the oral cavity.
(i) The premaxilla (intermaxillary segment), containing the incisor teeth, forms from the fusion of the globular processes of the median nasal process.
(ii) The palatal processes advance in a medial direction from the maxillary processes of the first branchial arch, fusing in the midline in an anterior-to-posterior sequence, and unite with the premaxilla and the developing nasal septum.
(iii) The soft palate forms from continued growth of the posterior edge of these palatal processes, ending with the formation and fusion of the two halves of the uvula.
(d) The nose originates in the cranial ectoderm, which subsequently develops into the frontonasal prominence, with the paired nasal placodes becoming recognizable during the third week of development.
(i) The superior portion of the nose is formed from the lateral nasal processes, whereas the inferior portion of the nasal cavity is incomplete until the paired maxillary processes of the first branchial arch grow anteriorly and medially to fuse with the median nasal processes.
(ii) The nasal cavities extend posteriorly during development influenced by the posteriorly directed fusion of the palatal processes, thinning out the membrane that separates them from the oral cavity.
(iii) By the 38th day of development, the two-layer membrane consisting of nasal and oral epithelia ruptures, and forms the choanae (posterior nares).
(a) Failure of such rupture results in choanal atresia, although these choanae are not in the same location as the definitive choanae, which will eventually be located more posteriorly; however, it does explain the unexpectedly anterior location given the eventual normal development of the choanae.
(i) The cranial base and nasal septum are in cartilaginous continuity, and fetal facial bones and teeth organize themselves around the cartilaginous nasal capsule.
(ii) The normal nasomaxillary complex grows both downward and forward.
D. The branchial apparatus
1. Branchial arches
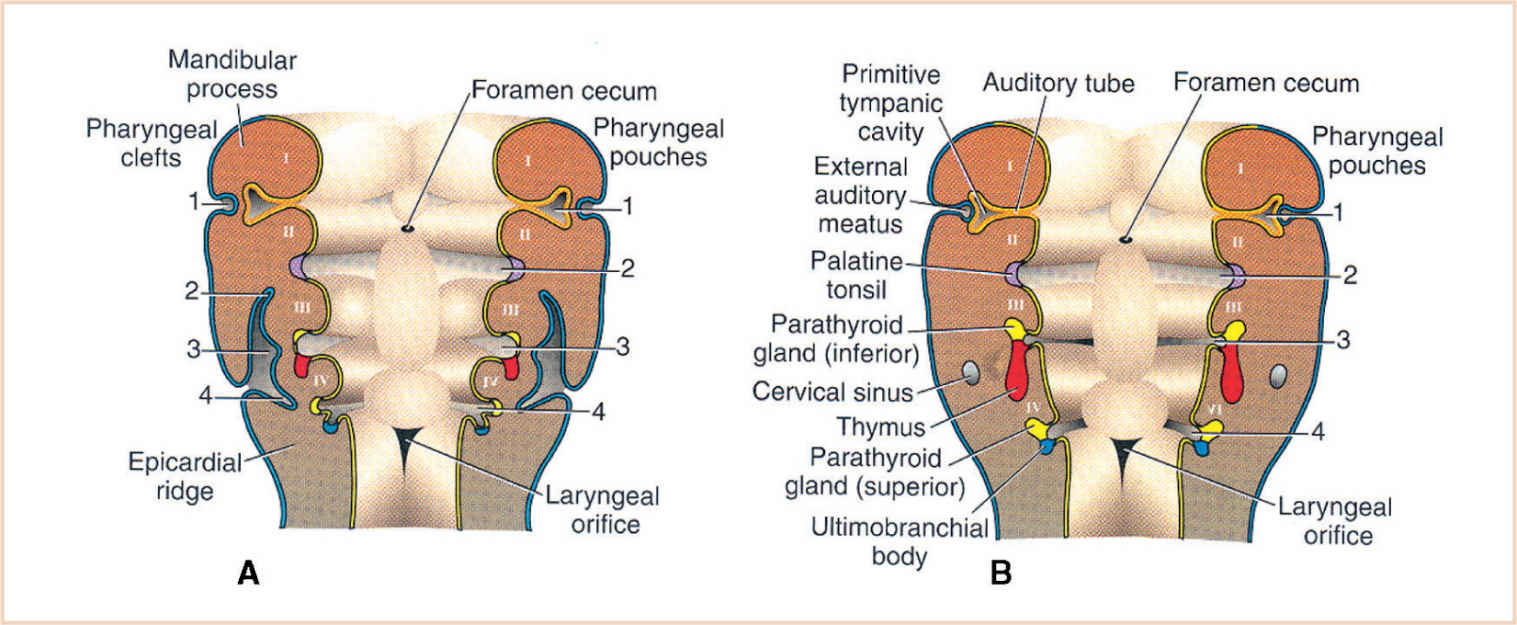
a. The branchial apparatus consists of four branchial arches visible on the surface of the embryo as well as a fifth and sixth arch that cannot be seen on the surface.
b. Branchial pouches and clefts are likewise numbered craniocaudally (Fig. 16.7).
c. The first branchial arch cartilage, known as Meckel’s cartilage, is the position of the future mandible, as well as the eventual malleus and incus, which develop by enchondral ossification.
(1) The sphenomandibular ligament remains from this embryological anlage, whereas the balance of the first arch cartilage disappears almost completely.
d. The second branchial cartilage produces the stapes, the styloid process, the stylohyoid ligament, and the superior portion of the body of the hyoid.
e. The other branchial arch cartilages contribute to the inferior portion of the hyoid as well as the thyroid cartilage.
f. Striated muscles are also formed in the respective branchial arch mesenchyme.
(1) Myoblasts differentiate and migrate to various parts of the head and neck, where they form the muscles of mastication and facial expression, each retaining their own original nerve supply.
(2) Although we think of muscular action in the head and neck as far removed from the origin and course of the cranial nerves, fetal nerves that supply the branchial arch derivatives have only a short distance to travel from the brain.
(3) The trigeminal nerve (V) supplies the skin covering the parts of the face derived from the first branchial arch via its maxillary and mandibular divisions (the ophthalmic division does not make a contribution).
(4) The facial nerve (VII) supplies the muscles derived from the first arch.
(5) The nerve of the third branchial arch is the glossopharyngeal (IX) cranial nerve.
(6) Two branches of the vagus nerve (X) supply the remaining branchial arches.
(7) The superior laryngeal nerve innervates derivatives of the sixth branchial arch.
2. Branchial pouches
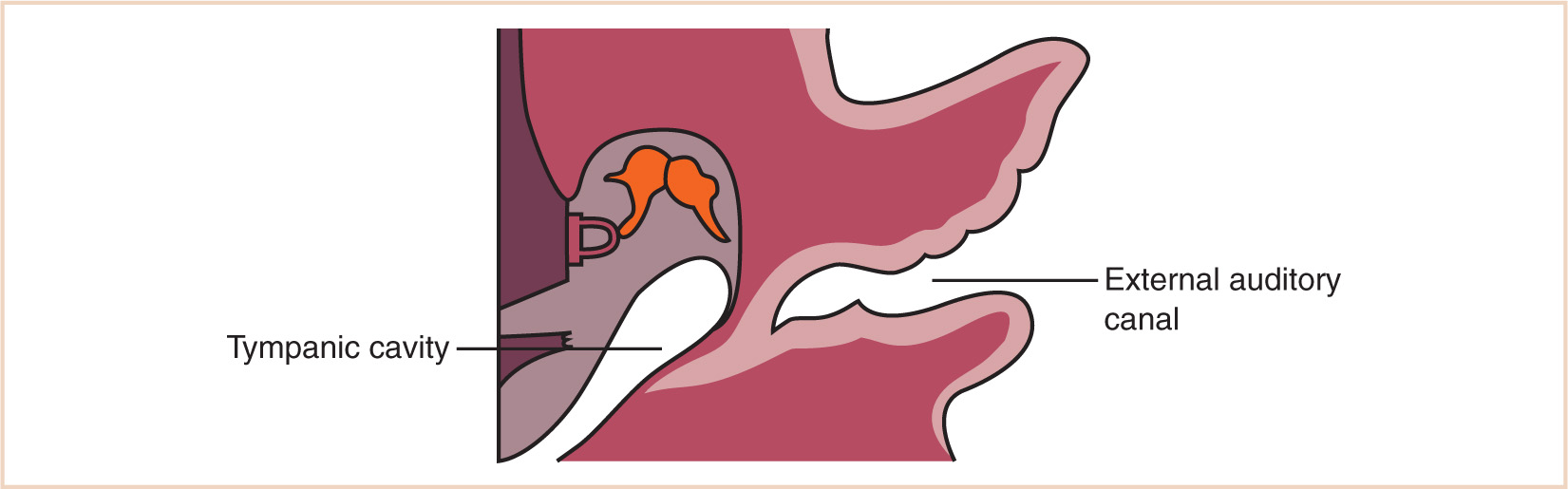
a. The first branchial pouch develops into the tubotympanic recess, becoming the auditory tube and the middle ear cavity (Fig. 16.8).
b. The cavity of the second branchial pouch is largely obliterated as the palatine tonsil develops, but part of it remains as the tonsillar fossa (intratonsillar cleft).
c. The endoderm of the second branchial pouch becomes the surface epithelium of the tonsil and the lining of its crypts, with the mesenchyme around the pouch differentiating into lymphoid tissue.
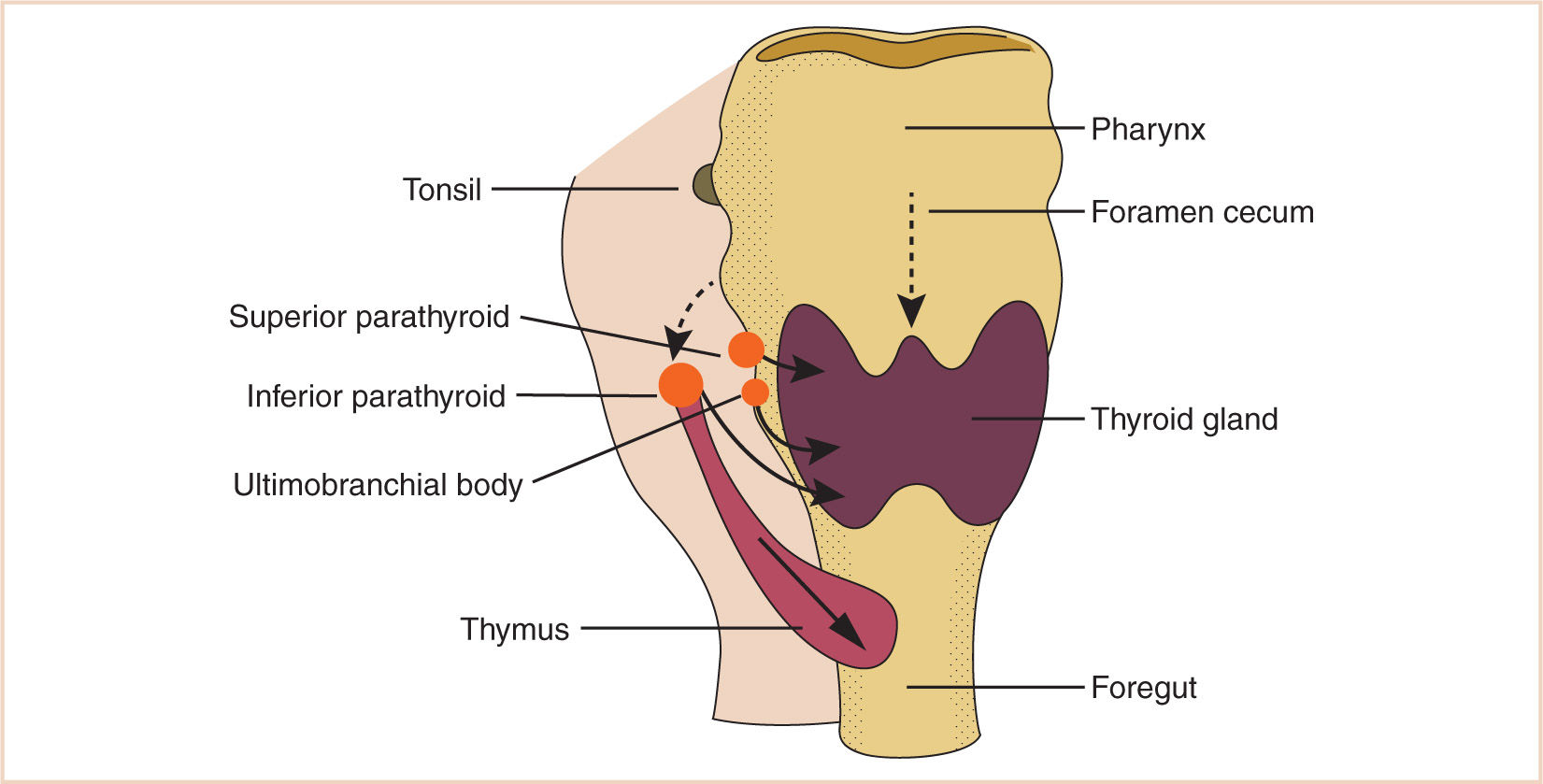
d. The endoderm of the dorsal part of the third branchial pouches differentiates into the inferior parathyroids, and the ventral parts unite to become the thymus.
e. The endoderm of the fourth branchial pouches differentiates into the superior parathyroid glands, and the ventral parts develop into the ultimobranchial bodies, the calcitonin-secreting portion of the thyroid.
3. Branchial clefts (Fig. 16.9)
a. The first branchial cleft (between the first and second arches) forms the external ear, which is the only normal structure to arise from a branchial cleft; however, if the second arch does not grow caudally over the third and fourth arches, as it normally should, then the second, third, and fourth clefts can remain as branchial fistulas, in contact with the skin surface between the clavicle and the mandible.
E. The thyroid begins as a thickening of the endoderm of the floor of the pharynx, in the midline between the first and second pouches, at the foramen cecum.
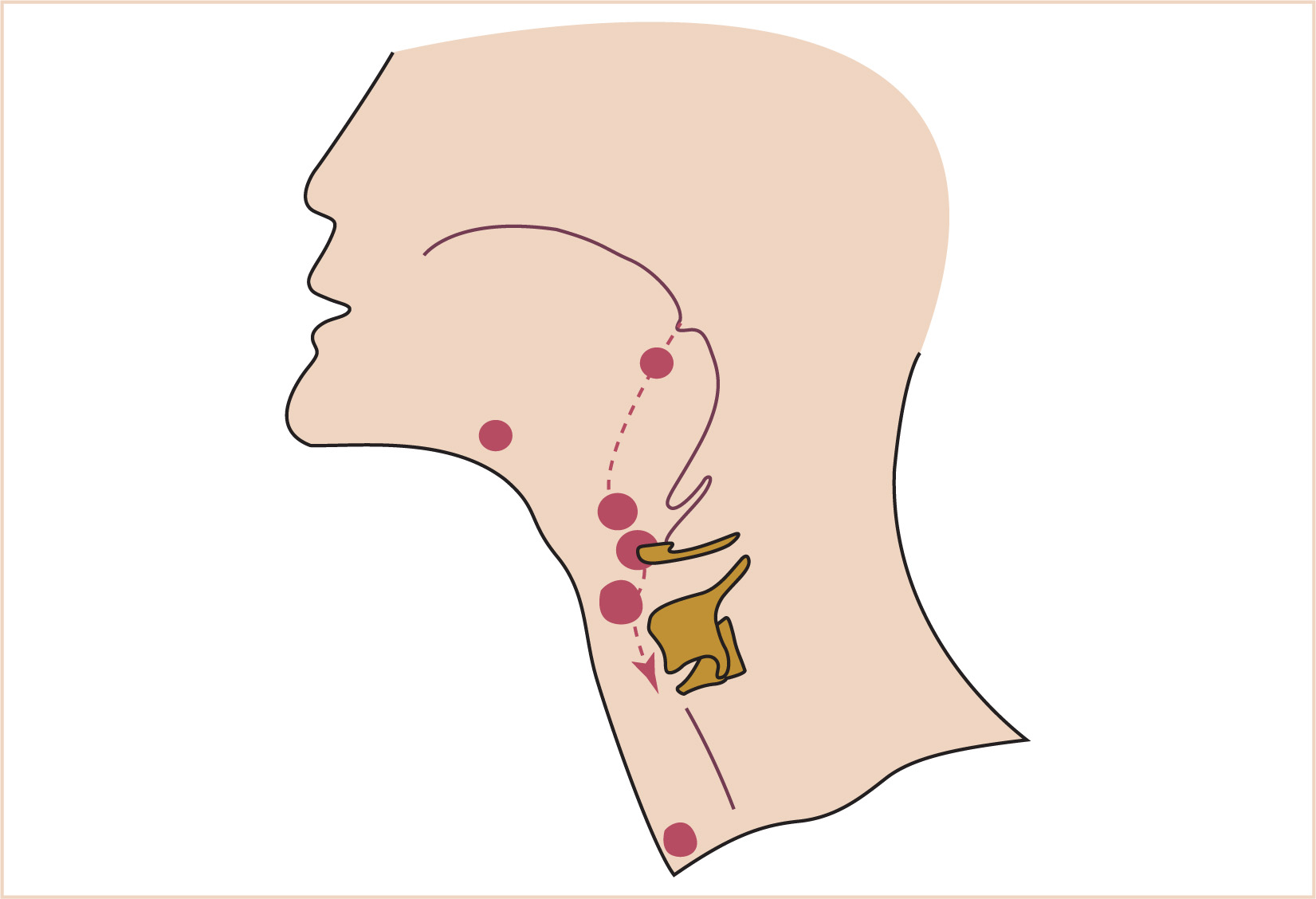
1. A thin connection, the thyroglossal duct, remains attached to the oral cavity, and its point of attachment marks the origin of the thyroid gland.
2. The thyroid descends along the thyroglossal duct and reaches the level of the first tracheal ring at about the 7th week of gestation (Fig. 16.10).
a. The thyroglossal duct is then normally obliterated.
b. Accessory thyroid tissue may be deposited anywhere along this path; on the other hand, failure of the thyroid to descend may result in a lingual thyroid.
F. The paired parathyroid glands develop from separate pouches, with one pair derived from the third and the other from the fourth branchial pouch.
1. At the seventh week of development, the parathyroid glands move caudally from their respective branchial pouches, with the parathyroids derived from the third pouch moving more caudally than the parathyroids of the fourth pouch.
2. Accessory parathyroid tissue may be left along the line of migration.
G. The larynx
1. Development of the respiratory system begins at approximately 3 weeks of gestational age with the formation of the laryngotracheal tube from the ventral wall of the foregut.
a. The normal laryngotracheal tube grows faster than the esophagus, which forms from the caudal growth of the foregut.
b. The laryngotracheal tube then grows caudally into the splanchnic mesoderm on the ventral surface of the foregut, dividing into the right and left lung buds.
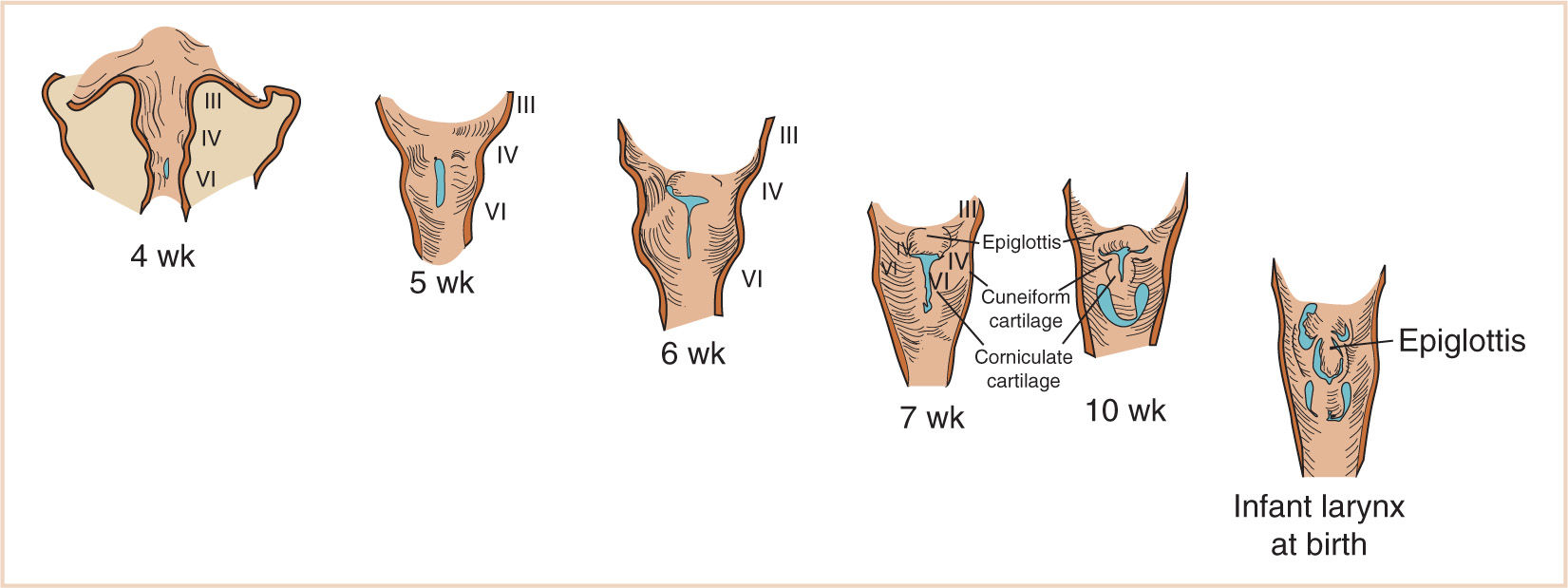
c. The epiglottis begins to form from the hypobranchial eminence of the third and fourth arches at approximately 30 to 32 days of gestation.
d. The anlage of the arytenoid cartilages can be identified on both sides of the laryngeal slit at this time and continue to grow during the fifth week of development.
e. The aryepiglottic folds develop from the lateral boundaries of the fourth arch along a line from the hypobranchial eminence (epiglottis) to the arytenoid eminence of the sixth arch.
f. Incomplete development at this stage may produce variable degrees of persistent laryngeal cleft (Fig. 16.24). A definite larynx may be seen by 41 days of gestation (Fig. 16.11).
2. The cricoid and thyroid cartilages begin to develop prior to the arytenoid cartilages and begin chondrification at about 7 weeks of gestation.
a. As the thyroid cartilages develop, the glottis deepens and the true vocal cords are aligned within the thyroid laminae.
b. Failure of the true vocal cords to split at 10 weeks of gestation to form the primitive glottis results in congenital atresia of the larynx or, more often, a complete or partial congenital laryngeal web.
c. Although webs may be supraglottic or subglottic, most occur at the level of the glottis.
d. Congenital cysts of the supraglottic region are possibly remnants of the third branchial pouch and lie superior to the derivatives of the fourth arch.
e. By the 10th to 11th weeks of gestation, the major structures of the larynx have developed and the cartilages are chondrifying.
f. If the separation of the esophagus and trachea is slightly delayed and the margins of the laryngotracheal groove fail to fuse adequately, the rapidly growing trachea separates the proximal and distal esophagus, resulting in the most common form of esophageal atresia and distal tracheoesophageal fistula.
II. Disorders of the head and neck
A. Craniosynostosis
1. Background
a. Deformation of the skull in the newborn may either be the result of premature closure of one or more of the cranial sutures or due to positioning of the fetus in utero.
(1) The term deformation is used to describe changes caused by positioning from premature suture closure, for example, deformational plagiocephaly.
(2) True cranial synostosis has an incidence of about 0.5%. Surgical correction of skull shape is done for cosmetic reasons except when brain growth is restricted from multiple suture closures.
(3) Deformational changes may be fixed nonsurgically by orthotic treatment using a helmet if done early enough.
2. Embryology/anatomy
a. In the fetus, the bones of the skull are derived from ossification centers within the fibrous desmocranium, which surrounds the developing brain. These centers act like tectonic plates whose contiguous boundaries become cranial sutures (Fig. 16.2).
(1) Brain growth pushes these plates apart, allowing new bone growth to occur along both sides of the suture line. In fact, it was in 1791 that skull growth was described to occur at the cranial sutures and that failure to grow at a particular suture lead to specific cranial deformity.
(a) Later, Virchow noted that growth was restricted in the perpendicular plane to the closed suture and enhanced in the parallel plane.
(2) The shape of the skull is described in specific terms:
(a) Scaphocephaly (premature closure of the sagittal suture) describes a long narrow skull shape.
(b) Brachycephaly (premature closure of the coronal sutures) results in a short but widened skull shape.
(c) Anterior plagiocephaly is closure of one side of the coronal suture.
(d) Posterior plagiocephaly is caused by closure of one of the lambdoidal sutures.
(e) Early closure of the metopic suture results in a peaked forehead called trigonocephaly.
(3) By 2 to 3 years of age, head growth approximates adult size, and by this time, a fibrous union has formed at the suture line, which continues until it ossifies at 6 to 8 years of age.
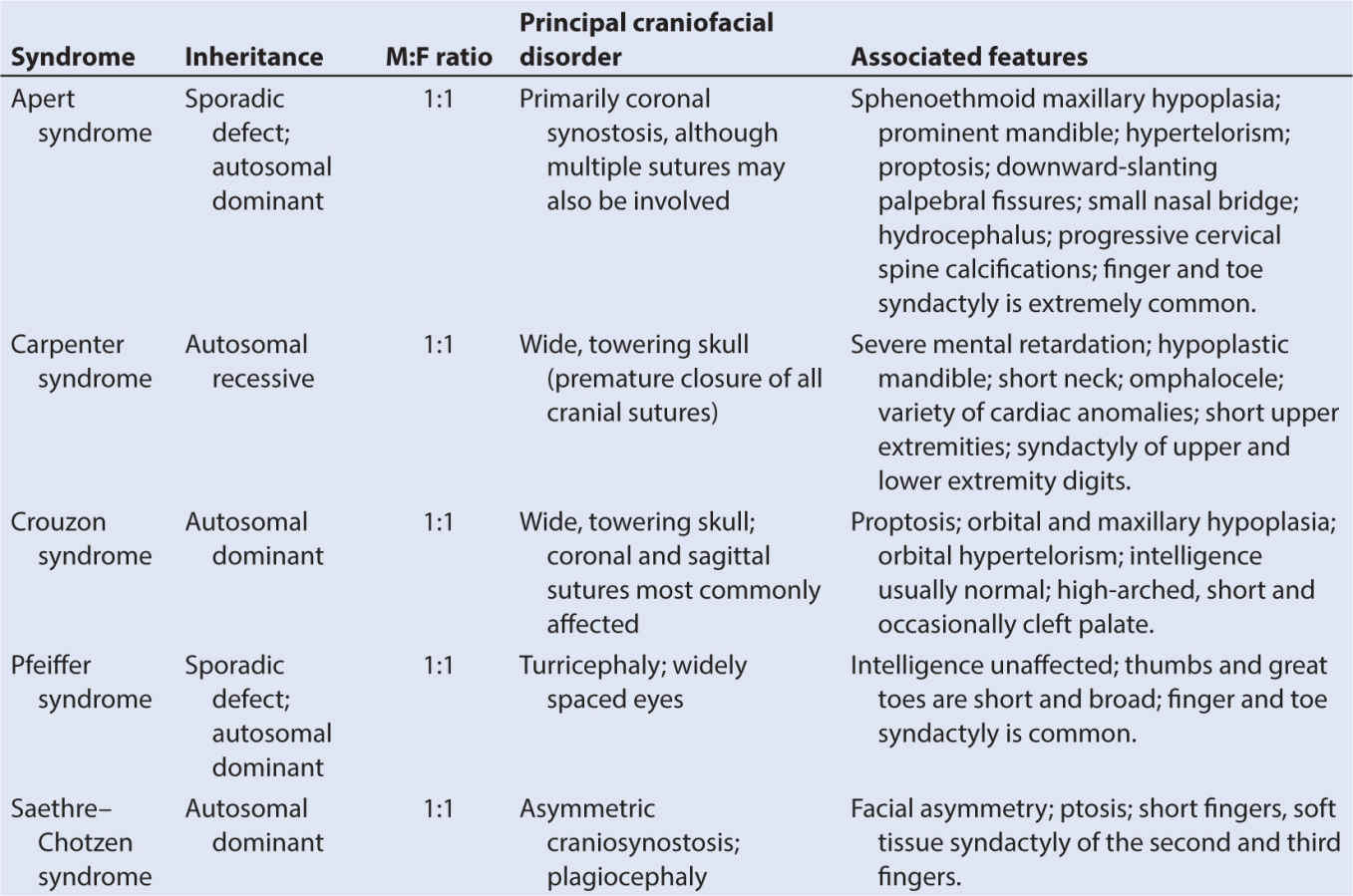
(4) Multiple suture closure is often associated with premature closure of the base of skull sutures. These are called complex craniofacial synostosis and are often syndromic in nature. Some of these syndromic synostoses, for example, Apert or Crouzon syndrome, have mutations of the genes involved with fibroblast growth factor receptor (FGFR).
3. Physiologic considerations
a. Closure of one or two sutures has no physiologic sequelae and is a cosmetic problem, although some would argue making a person look normal is not cosmetic.
b. Multiple suture closures on the other hand can restrict brain growth, resulting in increased intracranial pressure (ICP) and developmental delay. Surgery is performed to alleviate these issues.
c. In addition to increased ICP, complex synostosis patients may have other neurologic and airway problems, including the following:
(1) Shallow, misplaced orbits
(2) Midface hypoplasia
(3) Renal abnormalities
(4) Elevated ICP with hydrocephalus.
4. Surgical repair
a. One/two synostosis: If the child is under 3 months of age, surgical repair may be done endoscopically (Chapter 34). Endoscopic procedures can be performed rapidly, with few problems. Postoperatively these patients wear helmet orthotics to encourage symmetrical growth.
b. Open procedures are done for older children. Various calvarial remodeling procedures have been devised, including coronal craniectomy with frontal advancement, orbital rim alignment, and barrel staving, among others.
(1) A bicoronal, biparietal, or Meisterschnitt incision is used, enabling exposure of the anterior skull base, pterion, anterior fontanel, and the coronal sutures. For occipital synostoses, prone positioning with a biparietal or midsagittal incision is used. For multiple suture craniosynostosis, occasionally the entire cranial vault needs to be exposed, with a cheek and suboccipital headrest utilized (2).
(2) These procedures take longer, lose more blood, and have a longer convalescence. Blood loss can be minimized by the use of Raney clips along the skin edge and bone wax applied to bone edges.
(3) It is not unusual for these procedures to last 3 hours or more and require large amounts of fluid and blood.
(4) Blood-sparing techniques may be used to decrease the volume of blood transfused, thereby decreasing the chance of isoimmunization. Techniques that have been used include preoperative erythropoietin administration, intraoperative normovolemic hemodilution or blood salvage, and antifibrinolytic therapy. Of these, antifibrinolytic therapy has been proven to be efficacious and cost-effective.
(5) Careful intraoperative monitoring of hematocrit and electrolytes, especially calcium and potassium, is critical, particularly for large transfusion cases.
(6) Corneal shields are generally placed in the eyes and the eyelids sutured.
(7) Patients remain in hospital, often with significant postoperative swelling requiring perioperative intubation and nursing and respiratory care in the intensive care unit (ICU).
(8) In comparison, children undergoing endoscopic procedures have a relatively benign perioperative recovery with few, if any, problems. Spring-assisted surgery (SAS) has also been utilized to decrease the morbidity (invasive monitoring, transfusion requirements) of the larger, open procedures (3).
(a) Multiple/complex synostosis: These are not amenable to endoscopic repair. Major cranial or craniofacial procedures need to be done to correct these problems.
5. Anesthesia issues
a. Readiness for surgery
(1) Typical newborn anesthetic considerations apply for patients undergoing endoscopic surgical correction in the first several months of life.
(2) Multiple/complex synostosis patients may be syndromic, requiring attention to other comorbidities such as congenital heart disease, renal disease, etc.
b. Anesthesia goals
(1) For patients undergoing endoscopic repairs, the anesthetic technique should be directed to extubation at the end of the case.
(2) For large open procedures, maintain circulating volume and be prepared for postoperative ICU admission.
(3) Be vigilant for air embolism and massive blood loss.
c. General anesthesia
(1) Position: Supine for most open procedures, prone with head extended for endoscopic procedures (typically sagittal synostosis repair).
(2) Typical surgical time: 3 to 6 hours for open procedures, 1 to 2 hours for endoscopic procedures.
(3) Induction: Patient with normal airway—either inhalation or intravenous (IV) induction. Patient with history of difficult airway or anticipated difficult airway without prior surgery—inhalation induction with appropriate airway management equipment.
(4) Oral intubation is preferable because scalp reflection over the eyes and nose will interfere with access to a nasal tube.
(5) Note that midfacial hypoplasia, malar maldevelopment, and hypoplastic sinuses may result in impaired secretion clearance and difficulty with mask ventilation.
(6) A significant number of these children have sleep apnea and will develop supraglottic airway obstruction on induction and emergence (4).
(7) Monitoring: One large or two IV lines (especially for complex repairs), standard noninvasive monitoring, urinary catheter for cases over 4 hours, precordial Doppler, additional monitoring as needed, including laboratory monitoring for extensive procedures.
(8) Maintenance: Choice of anesthetic is dependent on the type of procedure and anticipated perioperative course.
(9) Watch for air embolism.
(10) Antifibrinolytic therapy stated with and initial bolus followed by a constant infusion. Various regimens have been used with bolus dose ranging from 15 to 50 mg per kg and infusion rates of 5 to 10 mg/kg/hour. The infusion is discontinued at the end of the procedure.
(11) Be mindful of massive blood loss; may be secondary to large resection, or as a complication of tearing the sagittal sinus during dissection.
(12) Cardiovascular collapse secondary to either of the above.
(13) Emergence: Patients repaired endoscopically may be recovered in the postanesthetic care unit (PACU). Patients with extensive open repairs will need ICU care postoperatively.
(14) Perioperative: Routine recovery for endoscopic surgery patients or noncomplex repairs. ICU with closer monitoring for complex repairs, including laboratory monitoring, adequacy of gas exchange, mechanical ventilation, and possible transfusion support. Cladis et al. have reported an incidence of postoperative hyponatremia of 30.6%, associated with increased preoperative ICP, blood loss, and female gender. Patients who received hyponatremic postoperative IV fluids were at greater risk (5).
B. Disease of the paranasal sinuses: Congenital malformations, inflammatory/infectious disorders, chronic sinusitis.
1. Background
a. Surgical treatment of paranasal sinus disease is used to drain infection, resect polyps, or perform a diagnostic evaluation for obstruction.
b. In almost all cases in pediatric patients, the procedure is performed to promote mucus drainage and promote collateral drainage due to the natural communication that exists between the paranasal sinuses.
2. Embryology/anatomy
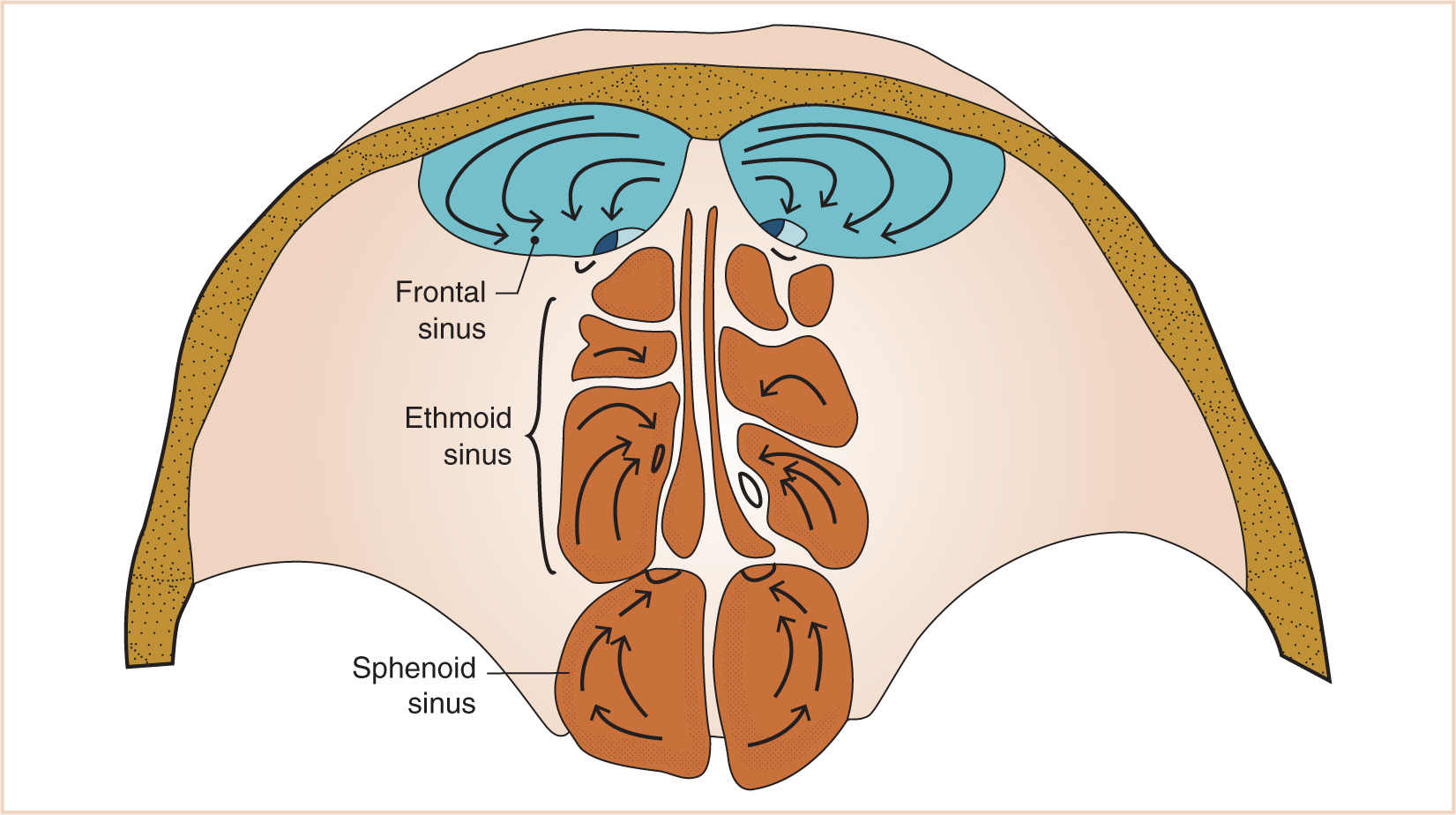
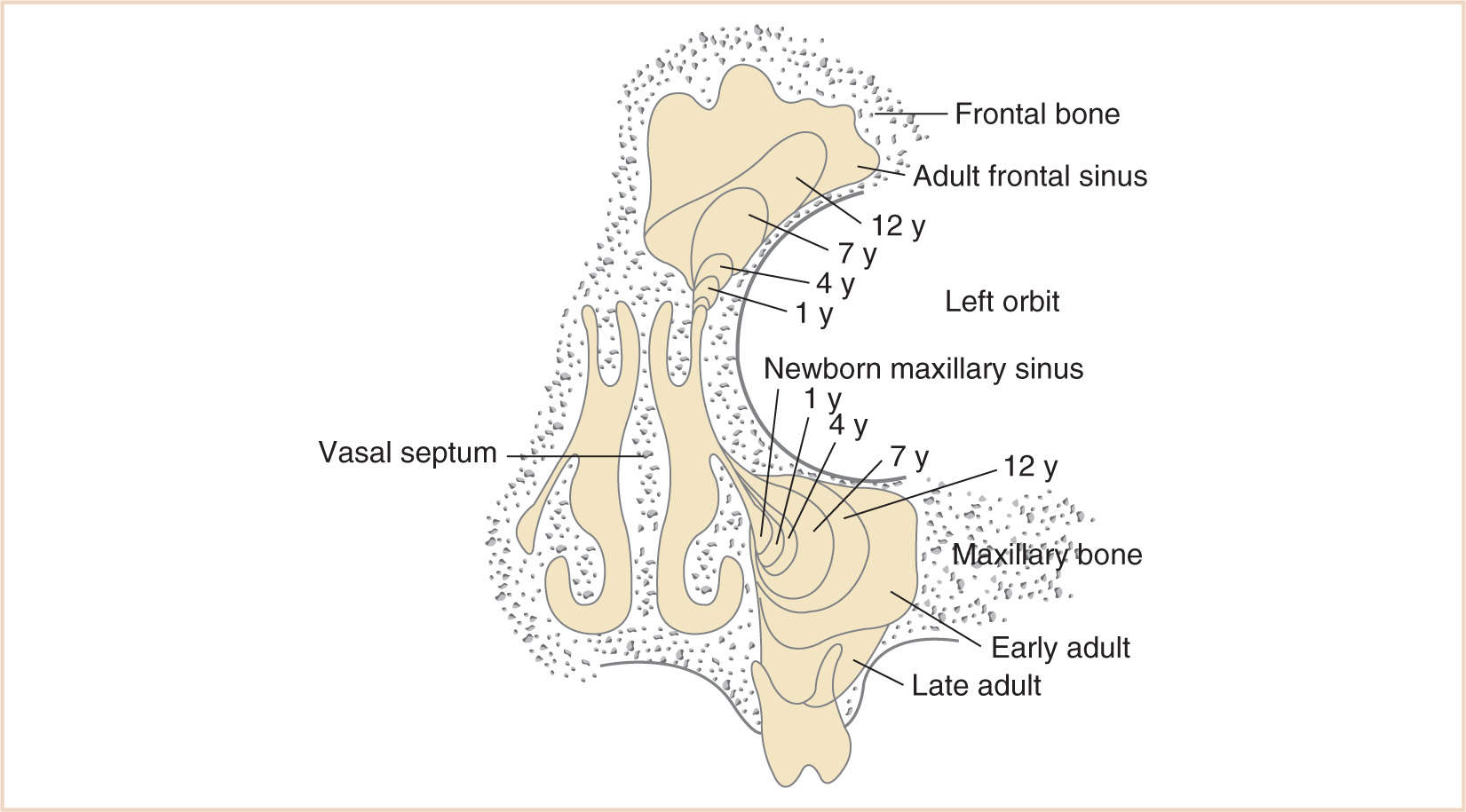
a. The paranasal sinuses begin their development at the end of fetal life, approximately 40 weeks of gestational age, from the mesenchyme of the lateral wall of the nasal cavity from which two horizontal grooves develop.
b. This maxilloturbinate mesenchyme grows medially into the nasal lumen as a precursor of the middle and inferior meatus and the inferior turbinate (Fig. 16.12).
c. The mesenchyme also begins to extend cranially to eventually develop into the middle and superior turbinates.
d. The completion of turbinate development signals the beginning of sinus development, which continues until early adult life (Fig. 16.13).
3. Physiologic considerations
a. The exact function of the paranasal sinuses is not well understood; however, inflammatory, infectious, and neoplastic diseases of the sinuses, because of their proximity to the brain, eyes, and aerodigestive tract, are of major significance to the anesthesiologist, particularly if there is functional impairment prior to anesthesia and surgery (Table 16.1).
4. Surgical repair
a. External approach: Caldwell-Luc: Intraoral incision in the gingival-buccal sulcus just posterior to the canine fossa; elevation of a submucoperiosteal flap (exposure of the infraorbital nerve); sinus is entered just inferior to the nerve, and diseased mucosa of the sinus is resected. A nasal-antral window is placed through the inferior meatus to allow additional drainage; gingival-buccal incision is then closed.
b. Endoscopic sinus surgery (Functional Endoscopic Sinus Surgery, or FESS): Following external or endoscopic sinus drainage, the nose/sinus is usually packed (oral airway).
5. Anesthesia issues
a. Readiness for surgery
(1) Adequate treatment of underlying medical conditions—antibiotics, steroids, and bronchodilators.
TABLE 16.1 Possible roles of the sinuses
1. To reduce the weight of the skull
2. To provide dampening pressure
3. Humidification and warmth of inspired air
4. Absorption of heat and insulation of the brain
5. Provision of sound resonance
6. Provision of mechanical rigidity
7. To increase the olfactory surface area
(2) Patients with severe respiratory disease (Kartagener syndrome, severe asthma, cystic fibrosis, etc.) may require pulmonary function tests, ECG, and echocardiogram evaluation for pulmonary hypertension and right-sided heart disease.
b. Anesthesia goals
(1) Minimize bleeding.
(2) Minimize coughing/bucking on emergence and in the postoperative period.
(3) Avoidance of nitrous oxide for patients with pulmonary hypertension.
c. General anesthesia
(1) Position: Semi-Fowler position; head up 30 degrees.
(2) Typical surgical time: 1.0 to 3.0 hours depending on complexity.
(3) Induction: IV or inhalation induction. Consideration must be given to adequate anesthetic depth on induction in order to minimize bronchoreactivity. Preoperative inhaler (sympathomimetic), use of IV steroid bolus, and anticholinergic should all be considered in order to attenuate bronchoreactivity.
(4) Monitoring: Standard noninvasive monitoring; additional monitoring based on length and complexity of surgery and associated medical problems.
(5) Maintenance
(a) Potent inhalation agent and/or narcotic technique.
(b) Avoidance of nitrous oxide for patients with pulmonary hypertension.
(c) Blood loss is moderate but not insignificant, rarely requiring transfusion.
(d) Vasoconstrictors such as topical oxymetazoline, phenylephrine, or cocaine are often used to decrease bleeding and may, because of systemic absorption, lead to intraoperative/perioperative hypertension, tachycardia, etc.
(6) Emergence: With nasal packing, an oral airway may be required for emergence and perioperative care.
(7) Perioperative
(a) Vigilance regarding adequacy of ventilation with nasal packing.
(b) Pain management can be accomplished with routine opioids.
(c) Nausea and vomiting risk with blood/mucus drainage in stomach.
(d) Airway irritability with coughing and/or bucking may increase sinus pressure and bleeding.
(8) Regional anesthesia: Sphenopalatine block (6) may be useful as an adjunct for perioperative analgesia. It can be approached intraorally at a transpalatal site just lateral to the vomer at the junction of the hard and soft palate where the anterior, middle, and posterior palatine nerves emerge. These nerves extend proximally to the sphenopalatine ganglion and the nasopalatine nerve. Analgesia of the upper teeth and gums, upper lip, cheek, lower eyelid, nasal ala and mucous membrane of the nose and nasopharynx, maxillary sinus, and soft and hard palate usually follow. Since vascular structures accompany the nerves, careful aspiration must be accomplished.
C. Maxillary deformity, midfacial retrusion, orbital abnormalities (Table 16.2).
1. Background
a. The face, specifically the maxilla and mandible, grows in a dynamic fashion throughout childhood under the influence of bony deposition and resorption, soft tissue contouring, and hormonal influences.
b. In a reciprocal fashion, the skull base or neurocranium influences midfacial development via the growth of the sinuses, which in turn, influence the skull base.
c. Displacement of these structures of the nasomaxillary complex occurs in horizontal, vertical, and anteroposterior axes.
d. These changes ultimately affect the proportions of the face and the morphology of all of the facial structures, including the upper airway.
2. Embryology/anatomy
a. While the mesenchyme of the maxilla develops from the maxillary division of the first branchial arch, it is influenced throughout its development by adjacent structures such as the skull base neurocranium and the nasomaxillary complex as well as complex interactive motion of the facial muscles and soft tissue contours (Fig. 16.14).
3. Physiologic considerations
a. Craniosynostosis, acrocephaly, midface hypoplasia, and syndactyly may have profound multisystem effects:
(1) Hypoplasia of upper airway structures may result in a small nasopharynx, choanal stenosis or atresia, hypoplastic, or nonexistent sinuses.
(2) Fusions of various cervical vertebrae may be present.
(3) Obstructive sleep apnea may result from chronic upper airway obstruction as a result of decreased available cross-sectional diameter of the airway, with a relatively large volume occupied by the tongue and pharyngeal musculature as well as an abnormally positioned palate.
(4) There is a 10% incidence of congenital heart disease.
(5) Cognitive impairment and developmental delay may result from elevated ICP if hydrocephalus is present. In addition, there is a high incidence of brain anomalies (agenesis of the corpus callosum, abnormal gyri).
(6) There is a 10% incidence of urogenital abnormalities (hydronephrosis, polycystic kidney disease, duplications, vaginal abnormalities).
(7) Syndactyly of fingers and toes, which may be partial or total, bony, or soft tissue.
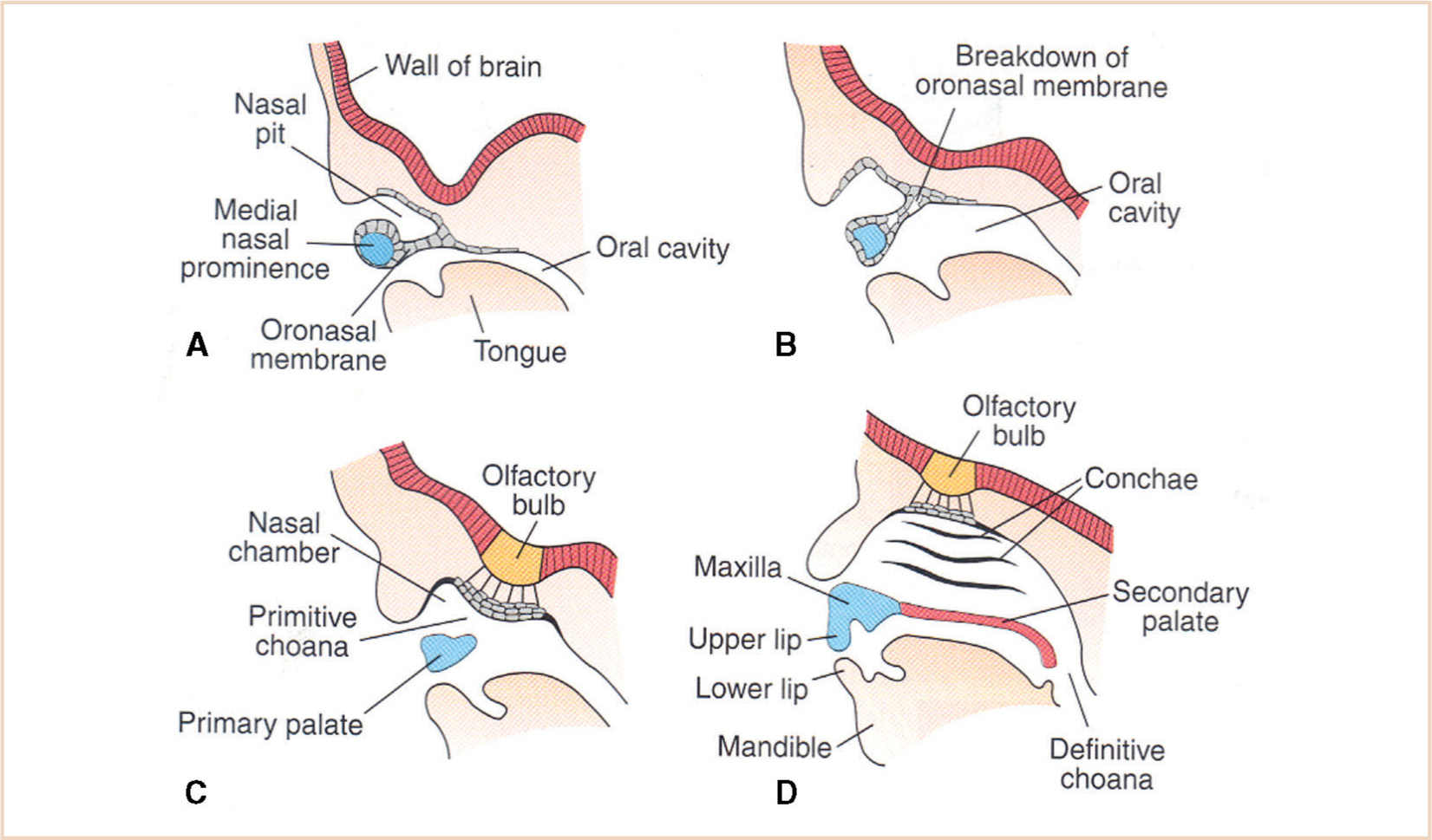
FIGURE 16.14 Development of the maxilla is influenced by contiguous structures—the skull base, the breakdown of the oronasal membrane, and the nasomaxillary complex. (A) 5 weeks, the primitive nasal cavity is separated from the oral cavity by an intact oronasal membrane; (B) 6 weeks, with breakdown of the oronasal membrane; (C) 7 weeks, with nasal and oral cavity communication; (D) 12 weeks, with an intact primary and secondary palate and patency of an appropriately developed choanal aperture.
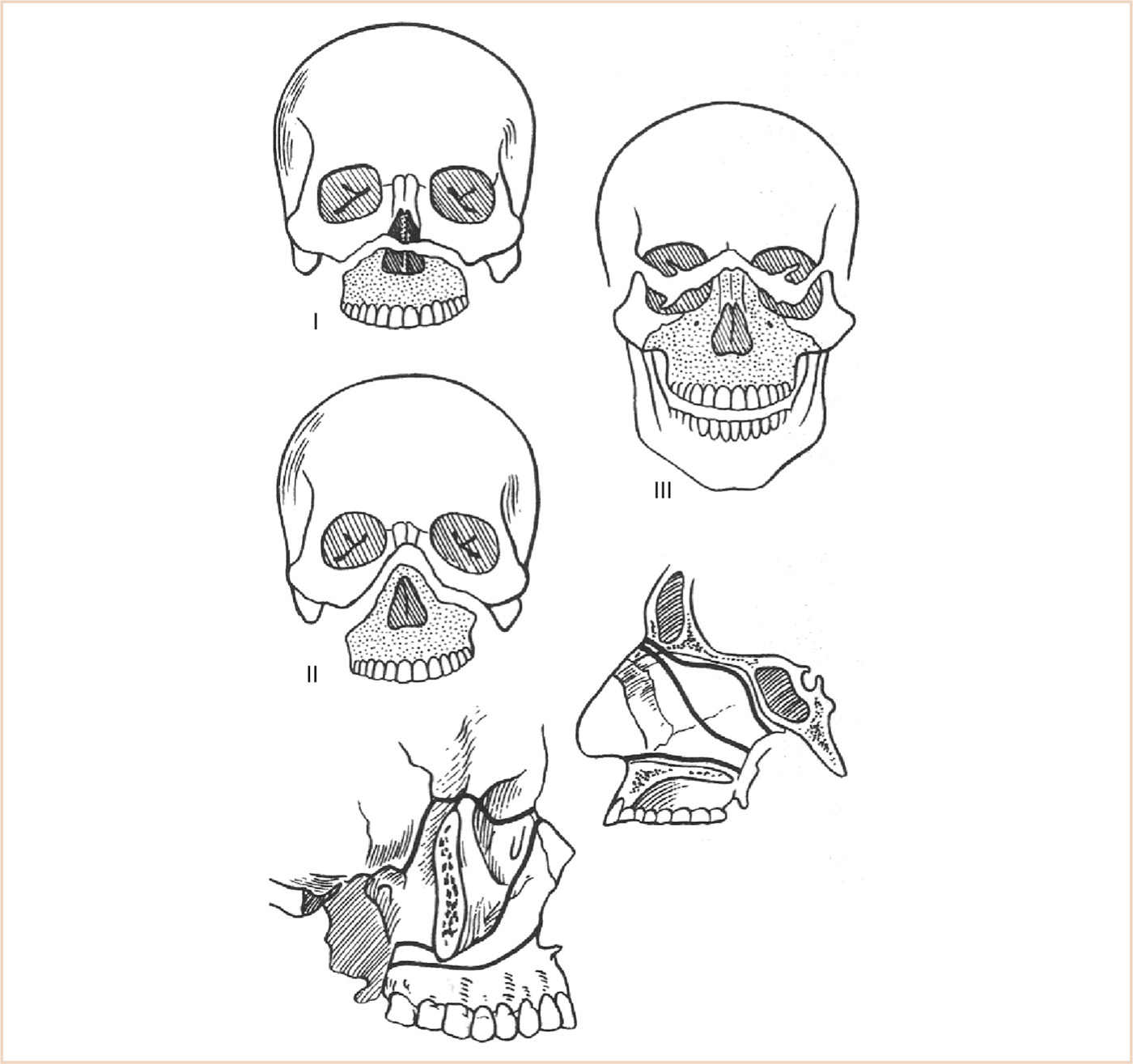
FIGURE 16.15 Left osteotomies LeFort I—transverse osteotomy superior to the teeth, to correct maxillary retrusion or vertical excess, traversing both maxillary sinuses and often into the nasal cavity. LeFort II—involves infraorbital incisions to create a pyramidal fracture. LeFort III—separation of the facial bones from the skull base.
4. Surgical repair (Fig. 16.15) (7)
a. The LeFort osteotomies are the basic surgical incisions that enable the surgeon to mobilize and manipulate the bony facial structures.
b. As human facial features result from the interaction of cranial, facial, and mandibular structures upon which the soft tissue is draped, the LeFort osteotomies by themselves are inadequate to totally correct or reconstruct facial problems.
c. They are therefore often combined with other maxillary or cranial procedures to achieve the desired result.
(1) LeFort I: A transverse osteotomy is made above the teeth via an intraoral vestibular incision.
(a) An osteotomy of the maxilla is then accomplished with a burr or a saw and completed with osteotomes.
(b) Both maxillary sinuses are osteotomized ± the nasal cavity.
(c) This effectively separates the palate and alveolar ridge from the rest of the upper face. It can then be advanced forward to correct for maxillary retrusion or cantilevered/tilted/widened to fix the bite. This is often done in conjunction with a mandibular osteotomy.
(2) LeFort II—this cut separates the maxilla and the upper midface from the rest of the face. It is rarely used as most structural problems incorporate the orbital and zygomatic areas.
(3) LeFort III—this osteotomy effectively separates the facial bones from the skull. Used with other procedures, it has an important role in the correction of many facial deformities. It may be accomplished from either an intra- or extracranial approach.
(4) These procedures have some limitations.
(a) All are accomplished by preserving the neurovascular bundle, which limits the maximum distance they can be mobilized.
(b) Another major limitation is the ability to stabilize the segments once they are moved.
(i) In the past, this was done by using bone grafts and wiring the segments together utilizing arch bars and wires in the mouth with circummandibular and circumzygomatic wires. Advances in microplating systems have made stabilization of bone segments less of an issue.
(ii) Screws and plates that are made of a resorbable polymer have replaced these metallic systems. Plating systems have allowed for exact and fairly rigid fixation of bone segments. This has eliminated the need for en-bloc wiring of the maxilla, mouth, and mandible.
(iii) While not eliminating the need for nasal intubation, the mouth is closed and fixed by using only elastics on the arch bars.
(iv) Bone grafts may still be needed to fill in large defects and build up certain areas.
(v) In addition to bone sources, synthetic material may be used for large defects or as onlay grafts. These materials can be shaped and sculpted to fit the needs of the patient.
(a) For the anesthesiologist, this en-bloc fixation requires a nasal endotracheal tube (ETT) for airway management.
(b) Intraoperative problems include blocked or kinked tubes with partial or complete transection of the ETT during osteotomies of the pterygoid bones.
CLINICAL PEARL Surgical repair of midfacial deformities requires a nasal ETT for airway management. Intraoperative problems include blocked or kinked tubes with partial or complete transection of the ETT during osteotomies of the pterygoid bones. Wire cutters need to be with the patient at all times to be able to cut the wires and emergently access the patient’s oral cavity for suctioning in the event of vomiting or the need to emergently reintubate.
(c) Wire cutters need to be with the patient at all times to be able to cut the wires and emergently access the patient’s oral cavity for suctioning in the event of vomiting or the need to emergently reintubate.
(5) Distraction osteogenesis has become a major technique in craniofacial surgery.
(a) The use of distraction allows the surgeon better control in positioning the bony segments after the osteotomy, enables the segments to be moved a greater distance, and eliminates the need for bone grafts.
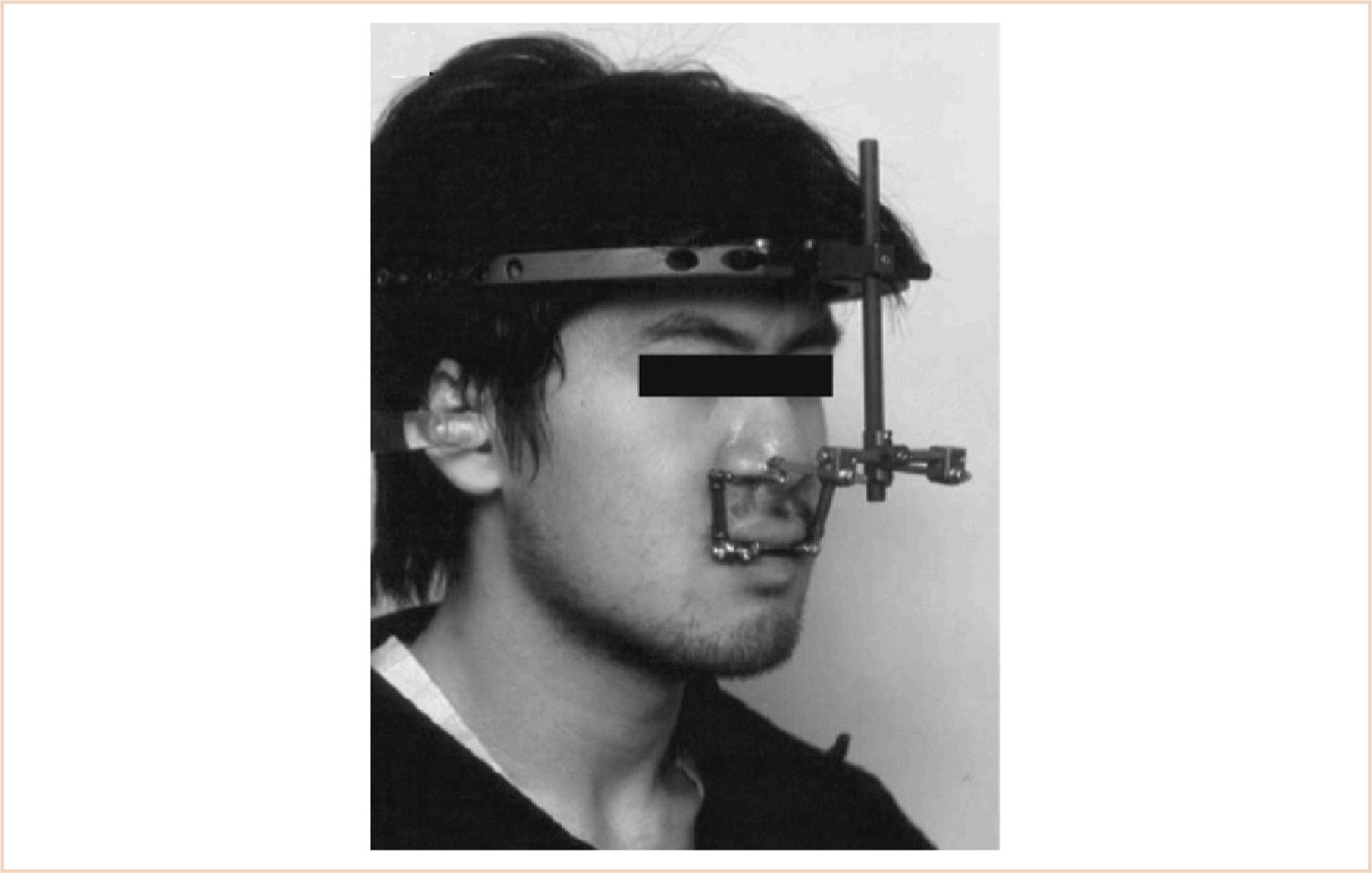
(i) Midface advancement using a halo distractor or rigid external distraction (RED) device is favored by some surgeons, while others may use an internal midface distractor.
(ii) Both types have drawbacks. For the anesthesiologist, the initial placement is not as much of a problem as the subsequent procedures to remove them.
(b) Limitation of mouth opening may occur once the distraction is finished.
(i) This may be the result of the new position of the maxilla in relation to the mandible, or may be caused from pain and swelling of the muscles through which the devices pass.
(ii) The anesthesiologist must therefore be prepared for a more difficult intubation following the initial procedure.
5. Anesthesia issues
a. Readiness for surgery
(1) The most important feature for the anesthesiologist’s readiness for surgery is understanding the extent of the surgical reconstruction contemplated.
(2) The most important feature for the patients’ readiness for surgery is the adequacy of their preoperative state and the stability of their comorbid illnesses.
b. Anesthesia goals
(1) Evaluate and plan for possible difficult mask fit due to midface hypoplasia, as well as possible difficult intubation. The mask fit is often more problematic than direct laryngoscopy and endotracheal intubation.
(2) Evaluate the cervical spine carefully for possible impaired range of motion and fusion abnormalities.
(3) The presence of a maxillary distraction device may make airway management more difficult (Fig. 16.16).
(4) Abnormal sinus and choanal development will make placement of a nasogastric tube or nasotracheal intubation difficult or anatomically impossible.
(5) Abnormal extremities may make vascular access more difficult.
(6) Consultation should be obtained for patients with comorbidities such as congenital heart disease, renal disease, neurological dysfunction, etc.
c. General anesthesia
(1) Position: Supine.
(2) Typical surgical time: Extremely variable depending on the extent of the procedure, anywhere from 2.5 to 3 hours to >8 hours.
(3) Induction
(a) Premedication must be carefully considered; these patients are often very anxious because of multiple prior procedures and office visits; on the other hand, they often do not handle their abundance of secretions well, and therefore deep sedation may result in salivation, coughing, and airway irritability even prior to induction.
(b) IV line placement may be difficult in the awake patient because of syndactyly; inhalation induction may be difficult because of the midface hypoplasia and difficulty with the fit of the mask.
(c) Following successful induction and endotracheal intubation, the tube is typically secured to the teeth by wire (or in edentulous patients, by circummandibular wire).
(d) Eye protection is usually provided with an ophthalmic ointment ± corneal guards and eyelid suturing by the surgeon.
(e) The operating table is typically rotated 90 degrees.
(4) Monitoring
(a) Significant blood loss (a portion of which will be occult because of loss into the stomach via the oropharynx) may accompany maxillary osteotomies, and adequate intravascular line placement for transfusion is critical.
(b) Depending on the extent of reconstruction, invasive arterial monitoring may be utilized.
(c) Difficulty in establishing peripheral vascular access may require placement of a central venous line for intraoperative and posteroperative care.
(d) Consideration should be given to precordial Doppler monitoring for air embolism.
(e) Additional monitors include urine output and temperature (patients often become hyperthermic because of heat retention and relatively little surface area exposure).
(5) Maintenance
(a) Antiemetics should be an integral part of the anesthetic plan because of the accumulation of blood in the stomach during surgery.
(b) Complex procedures, particularly those involving craniotomy, should be approached as neuroanesthesia procedures with the same considerations for prolonged brain exposure such as type and volume of IV fluid support, requirements for anesthetic depth, and mean arterial pressure (cerebral perfusion pressure) maintenance.
(c) Blood loss can be extensive (multiples of blood volumes for the more extensive and longer procedures), and the volume of blood lost is not always easy to appreciate because of insidious loss into the drapes and surgical field. Serial hematocrits are essential.
(d) Beware of coagulopathy for patients with large blood losses and hypothermia.
(e) Padding of extremities, joints, and subcutaneous nerves is critical for these prolonged procedures.
(6) Emergence
(a) Throat packs must be removed at the end of surgery.
(b) Patients with wire or banded maxillary fixation should be awakened fully. A wire cutter or scissor should be readily available in the OR and at the bedside throughout perioperative recovery.
(7) Perioperative: The location of perioperative care must be appropriate to the extent of the procedure and the concerns about comorbidities; patients may be recovered on the ward, step-down units, or ICU with or without continued mechanical ventilation for several days postoperatively.
(8) Regional anesthesia: Not applicable.
TABLE 16.3 Characteristics of cleft lip and palate patients
Incidence | 10:10,000 Caucasians |
| 25:10,000 Japanese |
| 3.3:10,000 Africans |
Gender | Male > female |
Isolated cleft palate | Female > male |
Isolated cleft lip | Unilateral 80%; bilateral 20% |
Cleft lip with associated cleft palate | 75% |
D. Congenital facial clefts of the lip and/or palate
1. Background
a. Cleft lip/palate (Table 16.3) is among the most common of congenital anomalies.
b. They may occur alone, as part of a syndrome (there are over 300 syndromes associated with facial clefting), or as a component of a sequence, for example, Pierre Robin sequence.
c. The repair of a cleft lip creates a normal facial appearance and normal psychological well-being. The repair of a cleft palate allows for normal vocalization without hypernasality and normal mastication of food.
2. Embryology/anatomy
a. The facial structures develop in the first 4 to 7 weeks of gestation from the frontonasal prominence and the paired maxillary and mandibular processes.
b. Development of normal maxillary structures involves both epithelial fusion and mesenchymal migration. The exact cause of facial clefting is unknown, but probably is the result of failure of one or both of these functions to occur.
c. Clefts of the palate may occur by the same mechanism as cleft lip or be secondary to anatomical obstruction, preventing the medial fusion of the maxillary processes.
d. The cleft palate associated with the Pierre Robin sequence occurs when the tongue, being displaced superiorly and posteriorly as a result of mandibular hypoplasia, interferes with palatal fusion.
3. Physiologic considerations
a. The facial area has numerous, interdependent physiologic functions which are not limited to breathing, eating, and communicating.
b. Hearing impairment may be the result of Eustachian tube dysfunction, requiring myringotomy and tubes.
c. Swallowing problems may also exist, contributing to aspiration and reactive airway disease.
d. Psychological well-being includes having normal facial structures. Dealing with these patients requires an understanding of how these psychological and physiologic issues interact.
4. Surgical repair
a. Cleft lip and palate: Surgical repair is directed not only toward approximating the tissue on either side of the cleft, but also rotating and advancing flaps for anatomic correction of the defect. Timing and sequencing of repair is still a matter of surgeon preference.
(1) One school of thought is to do primary repairs as early as possible in the newborn period.
(2) The other school of thought is to do staged repairs allowing for growth of muscle and alignment of alveolar segments to obtain the best functional repair. Sequential procedures consist of dentomaxillary appliance insertion, alveolar-lip adhesion release, cleft lip repair, and finally cleft palate repair.
(3) Pharyngeal flap and oronasal fistula repair may be needed subsequently for velopharyngeal insufficiency.
(4) Cleft palate alone: The infant need not undergo cleft palate repair as early as cleft lip repair, but should nevertheless undergo correction prior to initiation of speech.
5. Anesthesia issues
a. Readiness for surgery
(1) If the patient is a newborn while undergoing surgical procedures, all age-appropriate anesthetic considerations apply.
(2) Given the high incidence of associated syndromes and cardiac abnormalities, any patients with cardiac murmurs need to be evaluated prior to surgery. Antibiotic prophylaxis may be needed for any cardiac abnormalities found.
(3) Any history of apnea, breathing, or feeding problems may indicate airway problems necessitating alternative means of airway management.
b. Anesthesia goals
(1) Use oral RAE ETT for a low facial profile, out of the way of the surgical field.
(2) Anesthetic technique is chosen in order to have the patient breathing spontaneously and adequately assess depth of anesthesia.
(3) Extubate trachea when patient is fully awake.
c. General anesthesia
(1) Position: Supine, roll under shoulders; Rose position (supine with head over the edge of the operating table) is often utilized.
(2) Typical surgical time: 2 to 4 hours for unilateral cleft, 4 to 8 hours for bilateral cleft lip, and shorter for palate surgery.
(3) Induction
(a) Patient with normal airway—inhalation or IV induction; routine.
(b) Patient with history of difficult airway—inhalational induction with appropriate airway management equipment.
(4) Monitoring: Standard noninvasive monitoring, urinary catheter for cases over 4 hours, additional monitoring as needed depending on comorbidities.
(5) Maintenance
(a) Balanced anesthetic with potent inhalation agent plus narcotics. These patients need to be breathing spontaneously at the end of the case for safe extubation.
(b) Surgeons often use epinephrine-containing lidocaine during the case. Be mindful of local anesthetic dose.
(6) Emergence
(a) Surgeon may place a Logan Bow over the lip repair. Originally thought to decrease tension across the repair, it was found not to, and is primarily placed to protect the repair from accidental trauma. It may interfere with mask placement at the end of the case if the patient needs assistance.
(b) “Welcome” sleeves—arm splints for infants and small children, so they cannot put their fingers in their mouth and disrupt the repair.
(7) Perioperative
(a) Narcotic administration must be carefully titrated to preserve spontaneous respiration.
(b) Patient with ongoing airway obstruction problems may need to be transferred to an ICU setting for observation/management.
(8) Regional anesthesia
(a) Infraorbital nerve block (8) may be useful as either an alternative anesthetic technique or an adjunct to pain management. The infraorbital nerve provides sensory innervation to the upper lip and nose. The nerve exits from the infraorbital notch, which can be palpated along the inferior orbital rim and can be blocked as it exits. There is also a vein that travels with it which can be traumatized causing a black eye.
(b) Alternatively, it can also be blocked by approaching it from the alveolar sulcus subcutaneously. This works only at the beginning of the case as the surgeon dissects this area during the repair.
E. Velopharyngeal insufficiency
1. Background
a. Velopharyngeal insufficiency is the term used to describe hypernasal speech caused by inadequate separation of the nasopharynx from the oropharynx during phonation. Other signs include nasal emission and nasal turbulence (9).
b. It may be acquired or congenital, structural, or neuromuscular in etiology.
c. Closure of the cleft palate may result in insufficient tissue for development of normal length or function of the soft palate and require a posterior pharyngeal flap.
d. Different types of palatoplasties are performed to alleviate the hypernasality depending on the cause and dynamics of the insufficiency.
2. Embryology/anatomy
a. Facial structures develop in the first 4 to 7 weeks of gestation from the frontonasal prominence and the paired maxillary and mandibular processes.
b. Development of normal maxillary structures involves both epithelial fusion and mesenchymal migration.
c. Incomplete fusion/mesenchymal migration results in incomplete or submucous cleft of the palate typified by notching or a bifid uvula.
d. The velopharyngeal sphincter is composed of the five muscles in the soft palate and the superior constrictor of the pharynx.
e. The five soft palate muscles are the levator veli palatini, tensor veli palatini, musculus uvulae, palatoglossus, and the palatopharyngeus.
f. Surgical repair of the cleft palate involves approximating these various muscle groups in the midline to reconstitute the palate.
g. Any remaining velopharyngeal insufficiency may be remedied by posterior pharyngeal wall augmentation, pharyngeal flap, sphincter pharyngoplasty, or pushback palatoplasty (9).
3. Physiologic considerations
a. The facial area has numerous, interdependent physiologic functions which are not limited to breathing, eating, and communicating.
b. Hearing impairment may be the result of Eustachian tube dysfunction requiring myringotomy and tubes.
c. Swallowing problems may also exist contributing to aspiration and reactive airways disease. Psychological well-being includes having normal facial structures and ability to communicate. Dealing with these patients requires an understanding of how these psychological and physiologic issues interact.
4. Surgical repair
a. Pharyngeal flaps may be either superiorly or inferiorly based, although most are superiorly based as they have fewer complications.
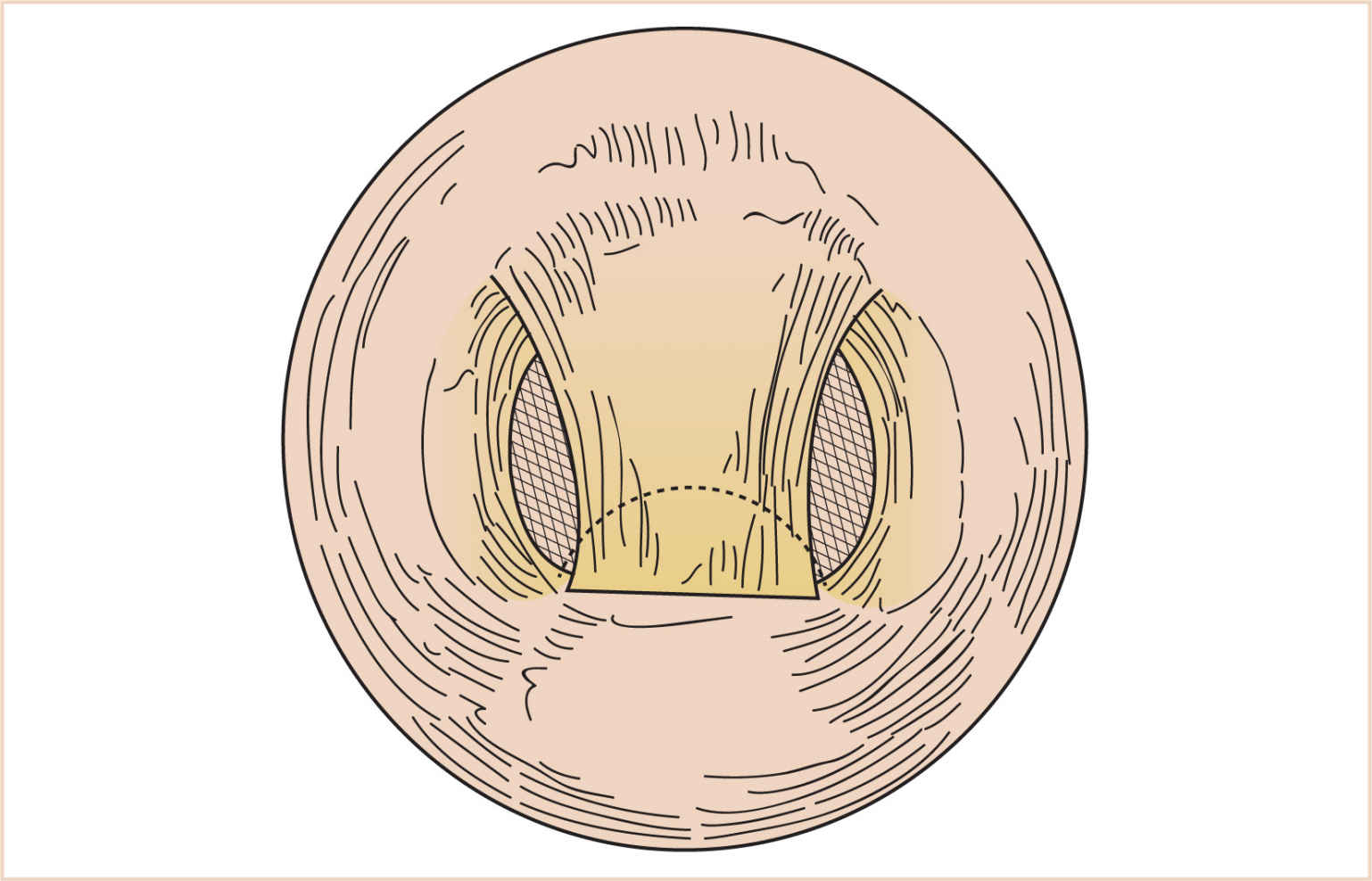
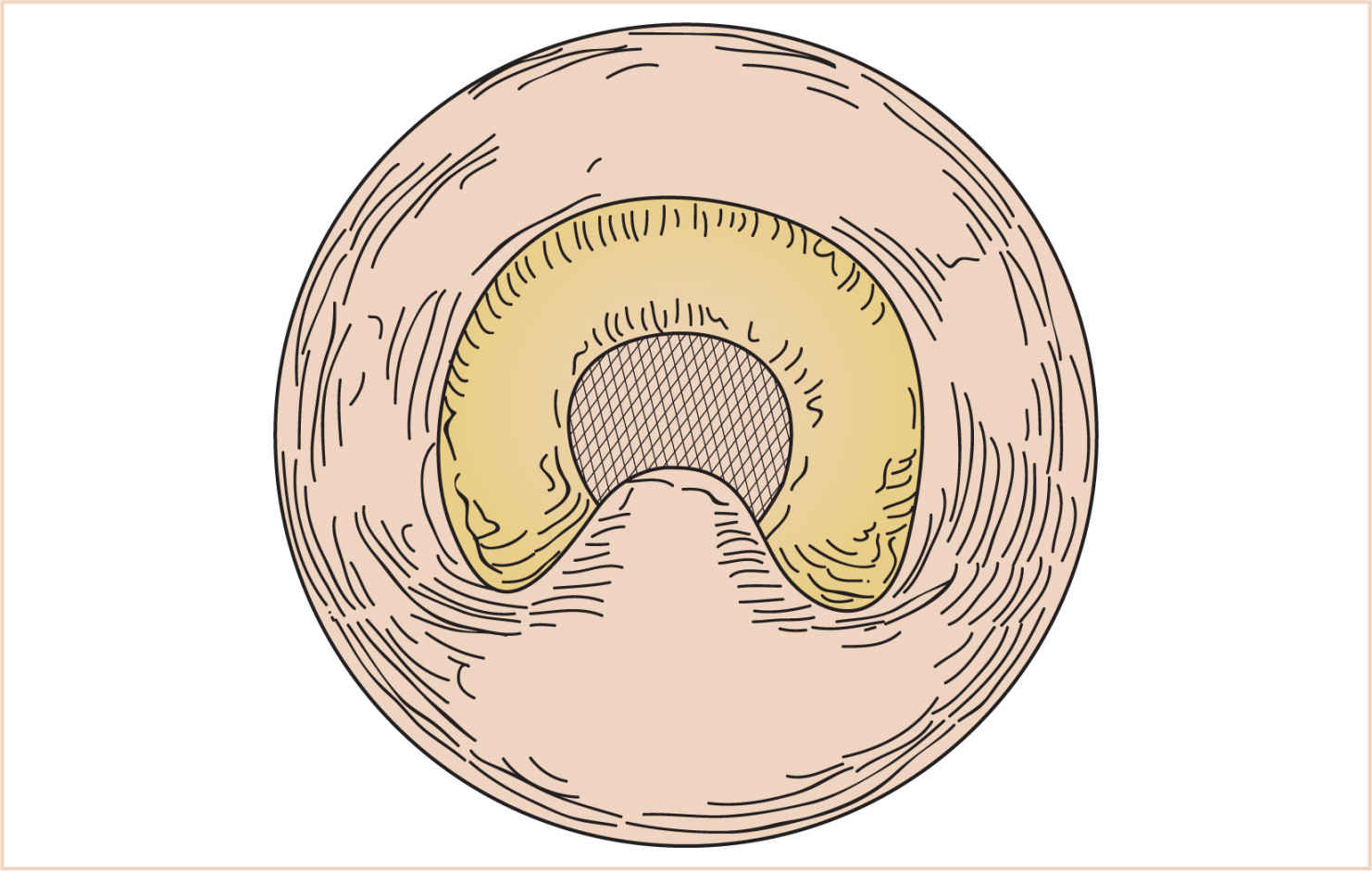
(1) A pedicle of mucosa is lifted off the posterior pharyngeal wall and swung upward and inserted into the soft palate, obturating the central portion of the velopharynx (Fig. 16.17). The length and width of the flap are tailored to the degree of velopharyngeal incompetence.
(2) Creation of a pharyngeal flap decreases the cross-sectional area of the airway to varying degrees. In some patients, this may change an adequate airway to a partially/totally obstructed one. These patients may exhibit hyperirritability or somnolence in the postoperative period. Return to the OR to take down the flap may be lifesaving in this situation.
b. A sphincter pharyngoplasty (Fig. 16.18) is accomplished by the creation of a vascularized and innervated myomucosal flap from the palatopharyngeus muscle and its mucosal covering. This flap then obturates the posterior and lateral pharyngeal walls, leaving a central passage. There may be less risk of airway obstruction and obstructive sleep apnea with this approach.
5. Anesthesia issues
a. Readiness for surgery
(1) Given the high incidence of related syndromes and cardiac abnormalities, any patients with cardiac murmurs need to be evaluated prior to surgery.
(2) Antibiotic prophylaxis may be needed for any cardiac abnormalities found.
(3) Any history of apnea, breathing, or feeding problems from the parents may indicate airway problems necessitating alternative means of airway management.
b. Anesthesia goals
(1) Safe induction and intubation.
(2) Anesthetic technique is chosen in order to have the patient breathing spontaneously and adequately assess depth of anesthesia.
(3) Extubate the trachea when patient is fully awake.
(4) Postoperative stay in ICU or closely monitored setting.
c. General anesthesia
(1) Position: Supine, roll under shoulders, table rotated 90 or 180 degrees.
(2) Typical surgical time: 1to 2 hours.
(3) Induction
(a) Patient with normal airway—either inhalation or IV induction.
(b) Patient with history of difficult airway—inhalation induction with appropriate airway management equipment.
(c) Oral RAE tube taped to the midline of the lower lip.
(d) Placement of Dingemann mouth gag.
(4) Monitoring
(a) Standard noninvasive monitoring, additional monitoring as needed during the case.
(b) Mandatory pulse oximetry postoperatively to detect hypoxia.
(5) Maintenance
(a) Inhalation agent plus narcotics. These patients need to be breathing spontaneously at the end of the case for safe extubation.
(b) Surgeons often use epinephrine-containing Xylocaine during the case—be mindful of local anesthetic dose.
(6) Emergence
(a) Surgeon may place a tongue stitch to pull tongue forward and off posterior pharyngeal wall to alleviate obstruction while emerging. This is helpful in patients who would be prone to airway obstruction from a preexisting cause, for example, Pierre Robin sequence.
(b) Patients with a pharyngeal flap can have significant airway obstruction postoperatively and may return for take down of the flap to relieve obstruction. Watch for signs and symptoms of airway obstruction.
(c) Avoid increased systemic blood pressure because it will increase bleeding.
(d) Avoid increased venous pressure (coughing, bucking, Valsalva) because it will increase venous bleeding, oozing, and cellular edema.
(7) Perioperative
(a) Narcotic administration must be carefully titrated to avoid respiratory depression.
(b) Patients need to be transferred to an ICU or similar setting for observation/management.
(c) Patients usually remain intubated for a day or two for pain management issues then extubated.
(8) Regional anesthesia: Not applicable.
F. Alveolar cleft and oronasal fistula
1. Background
a. Alveolar cleft and oronasal fistula repair are the final steps in cleft palate repair.
b. The alveolar cleft is the anatomic gap left in the alveolar ridge after the cleft palate has been repaired, whereas oronasal fistulas are tracts through the palate as the result of palate repair (Fig. 16.19).
2. Embryology/anatomy: Previously discussed.
3. Physiologic considerations
a. Physiologic consequences are minimal for these conditions.
b. These repairs are usually the last to be done, mostly after 9 years of age. Orthodontic procedures and prostheses can then be utilized.
c. The result is often improved self-image and social interaction.
4. Surgical repair
a. Alveolar cleft
(1) After the cleft palate repair, missing bone in the gum line may require further surgery. The “alveolus” is the tooth-containing bony portion of the gum line of the maxilla and mandible. The grafting of bone to this area is alveolar cleft bone grafting (ACBG) (Table 16.4).
(2) Filling the alveolar defect requires a bone graft usually taken from the iliac crest. This is done with the patient in lateral position. The iliac crest is typically utilized because cancellous bone transforms into alveolar bone postoperatively; other sites of cancellous bone such as the calvarium, mandible, and tibia are less commonly utilized.
(3) Some surgeons use fibrin glue prepared from autologous plasma and co-administered with thrombin, converting the fibrinogen to fibrin, to help stabilize the graft and promote bone healing.
(4) The patient is turned and redraped in the supine position for the repair.
(5) The alveolar cleft is filled with bone graft and fibrin glue.
(6) Finally, the area is covered with CoePak, gingival-periosteal packing, to protect the surgical area.
b. Oronasal fistula: Similar approach as cleft palate repair.
TABLE 16.4 Objectives of alveolar bone grafting
Fill residual osseous cleft of the alveolus and anterior palate
Give support to the alar base
Eliminate oronasal fistula
Eliminate mucosal recesses
Enhance maxillary stability
Consolidate the maxilla to facilitate subsequent surgery
Give bony support to adjacent dentition
Improvement of vestibular soft tissue relationships
Improve alveolar height and contour
Provide bone for subsequent implants
Improve dental and facial aesthetics
5. Anesthesia issues
a. Readiness for surgery
(1) By the time this procedure is scheduled, the patients are usually well known and there are few problems that have not been addressed previously. Past anesthetics should be reviewed.
b. Anesthesia considerations
(1) Same as those for cleft palate.
(2) Initially in lateral position for iliac bone graft, then supine.
(3) On emergence, ascertain ETT is not pressing against repair site.
G. Mandibular hypoplasia and Robin sequence
1. Background
a. Robin sequence is defined by micrognathia, glossoptosis, and airway obstruction.
b. Pierre Robin was the first to identify glossoptosis as the etiology of the airway obstruction.
c. Cleft palate occurs in 90% of Robin sequence patients.
d. Robin sequence may be part of a syndrome.
2. Embryology/anatomy—(see Mandibular Surgery)
a. Sequence (definition)—a pattern of anomalies derived from a single prior anomaly or mechanical factor.
b. Hypoplasia of mandible results in glossoptosis, causing airway obstruction.
c. Cleft palate result of failure of lateral ridges of palate to fuse secondary to mechanical obstruction of tongue.
3. Physiologic considerations
a. All physiologic considerations are the result of airway obstruction, including increased work of breathing, hypoxia, difficulty feeding, and failure to thrive.
b. All treatment modalities are aimed to relieve airway obstruction.
c. Medical treatment includes prone positioning and use of nasopharyngeal airway.
4. Surgical repair
a. Tongue-lip adhesion
(1) Tip of tongue is surgically adhered to lower lip pulling the tongue forward relieving the airway obstruction.
(2) Remain intubated in ICU postoperatively for pain management until ready for extubation.
(3) At approximately 6 months of age, the adhesion is undone and the cleft palate repaired if one exists.
b. Mandibular distraction
(1) Bilateral mandibular osteotomies are done and bilateral external mandibular distraction devices placed.
(2) The infant remains intubated in the ICU as the mandible is distracted.
(3) The ETT is removed after approximately 1 week.
c. Tracheostomy
(1) Reserved when all other modalities fail.
(2) Comes with potential complication both medically and developmentally.
5. Anesthesia issues
a. Readiness for surgery
(1) If failure to thrive, infant may need nasogastric or G-tube feeds to get adequate calories.
(2) Evaluate concomitant problems if associated with a syndrome.
b. Anesthesia goals
(1) Maintain an adequate airway at all times.
(2) Postoperative management in ICU.
c. General anesthesia
(1) Position: Supine.
(2) Typical surgical time: 1.0 to 3.0 hours.
(3) Induction: A full discussion of the management of the difficult airway is not possible here, but any technique that allows the infant to breath spontaneously without airway obstruction can be used. These include use of a nasopharyngeal airway for insufflation or laryngeal mask airway. Tracheal intubation is best accomplished using a fiberoptic bronchoscope for nasal intubations while a video-laryngoscope, for example, glideslope, may be used for oral tubes.
(4) Monitoring: Standard noninvasive monitors.
(5) Maintenance: Inhalational anesthesia with narcotics.
(6) Emergence: Transport to ICU intubated.
(7) Perioperative: The airway can be “lost” at any point from
(a) Dislodgement of ETT—loss of CO2 with loss of inspiratory pressure
(b) Mainstem intubation especially during flexion—increase in peak inspiratory pressure (PIP) and decrease in CO2 waveform
(c) Plugging of ETT—very high PIP with little or no CO2
H. Diseases of the tongue: Lingual tonsil; congenital malformations; neoplastic disease; airway issues regarding tongue size to volume of oral cavity; muscular dystrophies.
1. Background
a. Most congenital lesions are nonmalignant and require excisional biopsy.
b. Neoplastic lesions require extensive resection and may have courses of postoperative irradiation and/or chemotherapy.
2. Embryology/anatomy
a. The tongue is composed of tissue from three embryonic sites (Fig. 16.5). The anterior two-thirds is derived from first arch mesenchyme, while the posterior portion is from the third and fourth arches.
b. Most of the muscle mass is derived from occipital somites. Nervous innervation comes from the 7th, 9th, and 12th cranial nerves.
c. In its normal state, the tongue does not quite fill the entire oral cavity. Any disease state that alters the ratio of tongue mass to oral cavity volume can lead to problems with breathing and eating.
d. Tongue mass may be increased primarily (e.g., Beckwith–Wiedeman syndrome) or secondarily (e.g., mucopolysaccharidoses).
e. Small tongues may be the result of hemifacial atrophy (Romberg syndrome) or congenital facial diplegia (Mobius syndrome).
f. Since the tongue is composed of striated muscle, various muscular dystrophies may present with fasciculations of the tongue, tongue atrophy, or muscular pseudohypertrophy.
g. Any of the neoplasms associated with striated muscle may also occur.
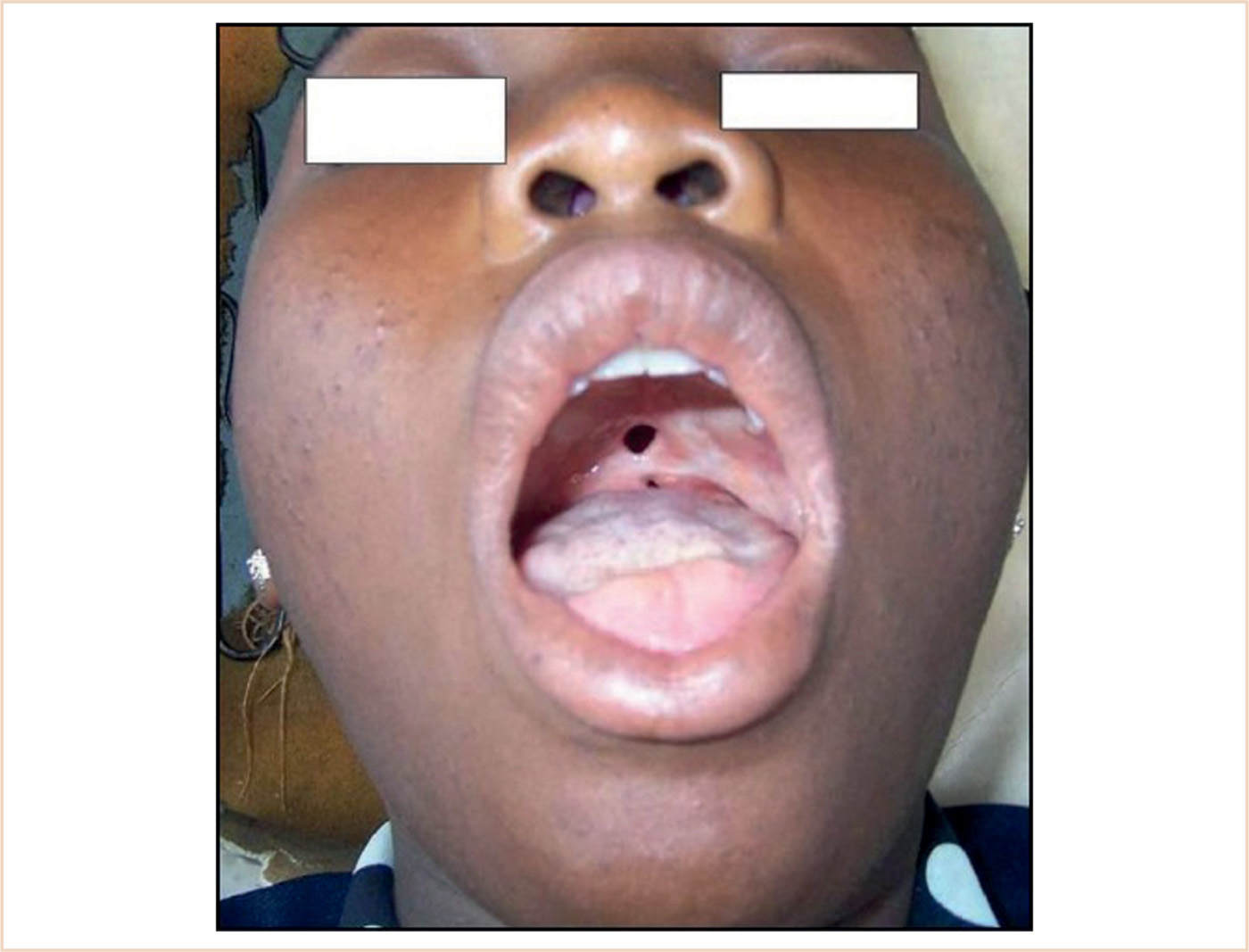
h. While not part of the immunologic system, the tongue base does have lymphoid tissue within it and is part of Waldeyer Ring. An enlarged lingual tonsil may be the cause of an unanticipated difficult intubation.
i. Ankyloglossia (tongue tie)—abnormal development of the lingual frenulum. Normally, cells surrounding the lingual frenulum degenerate during development, and the frenulum is the only residual connection of the tongue to the floor of the mouth. Failure of normal cell degeneration results in remnants of this anchoring tissue, which may extend to the tip of the tongue.
3. Physiologic considerations
a. The tongue is a complex organ involved with the senses of taste and speech, eating, and swallowing.
b. Most physiologic considerations related to anesthesia are limited to problems that contribute to aspiration, cause obstructed respiration, and impair tracheal intubation.
c. Chronic airway obstruction may lead to obstructive sleep apnea and cor pulmonale.
d. Inability to feed well may contribute to malnutrition and secondary problems.
e. Patients with Beckwith–Wiedeman syndrome experience hypoglycemia that needs treatment in the newborn period; this resolves as they mature.
f. Patients with muscular dystrophies will have other physiologic abnormalities particular to the type of dystrophy.
4. Surgical repair
a. Ankyloglossia: Correction usually involves snipping the short membranous frenulum. Rarely, a frenuloplasty will be needed to increase mobility. Local anesthetic infiltration will suffice as an analgesic for most cases.
b. Glossectomy (partial; total; volume reduction surgery for hypertrophy due to cystic hygroma; neoplasms). A Dingemann mouth gag may be used by the surgeon to facilitate exposure. Depending on the site and extent of the resection, elective tracheostomy may be performed.
5. Anesthesia issues
a. Readiness for surgery
(1) Correction of any comorbid physiologic conditions.
(2) Preoperative evaluation of the airway with CT scan/MRI or soft tissue neck films may be necessary.
b. Anesthesia goals
(1) Safe induction and tracheal intubation in light of possible difficult airway
(2) Safe tracheal extubation
c. General anesthesia
(1) Position: Almost always supine.
(2) Typical surgical time: May be extremely short (frenulectomy) to hours (partial glossectomy/tongue volume reduction surgery).
(3) Induction
(a) With normal anatomy, standard induction techniques will suffice.
(b) Be prepared to manage difficult airway with inhalation induction, awake sedate intubation, or awake sedate tracheostomy under local anesthesia.
(4) Monitoring: Standard monitoring unless invasive cardiovascular monitoring needed for secondary cardiac issues.
(5) Maintenance
(a) Nasotracheal intubation may be easier than oral, or it may be preferred if the patient will remain intubated postoperatively.
(b) A drying agent such as glycopyrrolate may be helpful to reduce oral secretions.
(c) A nasogastric tube to drain secretions and allow for perioperative enteral alimentation is needed if the patient will remain intubated for several days.
(d) Steroids may help to reduce tongue swelling postoperatively.
(6) Emergence
(a) No specific emergence considerations if extubation is planned and the patient has minimal swelling following the surgical procedure.
(b) Postoperative sedation for anticipated postoperative intubation.
(c) If a tracheostomy is performed as part of the procedure, consideration should be given to avoiding positive pressure ventilation postoperatively in order to decrease the chance of subcutaneous emphysema.
(7) Perioperative
(a) The tongue will respond to any incision by swelling, sometimes massively.
(b) Postsurgical swelling of lingual tissue usually takes 4 to 6 days to resolve. Postoperative ICU stay and prolonged intubation should therefore be anticipated.
(c) For the difficult reintubation patient, a tube exchanger at the bedside may be lifesaving.
(8) Regional anesthesia: Local anesthesia.
I. Diseases of the nose: Tumors of the anterior skull base and nose; nasopharyngeal tumors; juvenile nasal angiofibroma.
1. Background
a. Tumors in the area of the nasopharynx are rare in children and devastating when diagnosed. Improvements in surgical techniques, collaborative procedures among various specialties, and advances in skull base surgery now allow block resection of many of these tumors (Table 16.5).
b. As an example (with similar considerations for other nasopharyngeal tumors), juvenile nasal angiofibroma is the most common form of benign nasopharyngeal tumor in children, with an incidence of 0.16 to 2:10,000.
c. The typical age range at onset of symptoms is between 7 and 21 years.
d. Patients are almost always male.
e. In general, nasal angiofibromas account for 0.5% of all head and neck tumors, while malignant tumors account for 1% of childhood malignancies (10–12).
2. Embryology/anatomy
a. The nasopharynx and skull base are derived from ectodermal and mesenchymal cells, which form the structures of the head and neck.
b. Originally thought to arise from embryonic cartilage between the occipital skull base and sphenoid bones, nasal angiofibromas are now thought to arise from the posterolateral wall of the nasal cavity surrounding the sphenopalatine foramen, and histologically are composed of benign vascular and stromal tissue.
c. A network of vascular channels permeates the fibrous stroma, and because these channels do not have contractile elements, massive hemorrhage following biopsy or dissection is not uncommon.
d. Juvenile nasal angiofibromas originate from the superior margin of the sphenopalatine foramen and may extend posteriorly (into the nasopharynx), anteriorly (into the nasal cavity), and laterally (through the pterygopalatine fissure into the pterygomaxillary fossa).
TABLE 16.5 Tumors of the anterior skull base
Angiofibroma
Osteoma
Craniopharyngioma
Olfactory neuroblastoma
Chordoma
Chondrosarcoma
Rhabdomyosarcoma
Embryonal
Alveolar
Undifferentiated
Nasopharyngeal carcinoma
Squamous cell carcinoma
Nonkeratinizing carcinoma
Differentiated nonkeratinizing carcinoma
Undifferentiated carcinoma

Full access? Get Clinical Tree