Fig. 53.1
Dr. Ralph Waters at age 65 in 1948 from the same group picture that included Lucien Morris (Fig. 53.2). (From the personal collection of the author)
In my second year at Madison (Fig. 53.2), I participated in the ongoing departmental study of chloroform, which was subjected to scrutiny as though it were a brand new anesthetic agent [1]. I worked closely with Dr. Waters on the chloroform cases, becoming increasingly frustrated by the poor control over chloroform vapor concentration, and lamenting in the operating room that “anyone ought to be able to make a better vaporizer than this”. Several weeks later, while in Florida, Dr. Waters sent a postcard to the staff and residents with a single question: “Has Morris made a new vaporizer yet?” With that encouragement, I set out to solve the problem.

Fig. 53.2
A young Lucien Morris from a group photograph of his residency mates and the faculty at the University of Wisconsin. (From the personal collection of the author)
Though not known by me at the start of this quest, John Snow (1813–1858) had noted the importance of several principles that I eventually applied. He observed that there had to be an anesthetic surface area sufficient to ensure equilibrium of anesthetic with the air passing through it. He knew that temperature affected vaporization and to stabilize temperature he made his vaporizer of copper and surrounded it with a water jacket (water because it has a great capacity to hold heat) (see Fig. 52.2). But his and the contemporaneous vaporizer developed by Joseph Thomas Clover (1825–1882) were “non-rebreathing” systems that lacked the economy needed in modern vaporizers [2,3]. Other vaporizers developed in the last half of the nineteenth century had one or more deficiencies. Vernon Harcourt (1834–1919) for example, built an accurate chloroform vaporizer that was easily broken and its glass components did not lend themselves to temperature stability (Fig. 53.3).

Fig. 53.3
The Harcourt vaporizer was designed to produce a known delivered concentration of chloroform. Two glass bulbs, probably long since broken, were attached to the two arms. The vaporizer compensated for the effect of the temperature of the chloroform on the concentration produced. (Courtesy of the Wood Library-Museum of Anesthesiology, Park Ridge, IL)
In the first half of the twentieth century, volatile anesthetic concentrations were often provided to patients from gauze masks onto which the liquid anesthetic was dropped. There was no way to estimate the anesthetic concentration breathed by the patient. Alternatively, anesthetic concentrations were produced by anesthetic machines with metered gas flows to which anesthetic vapor was added. In these machines, vapor was added by diverting a coarsely variable portion of the total flow of gases breathed and rebreathed by the patient over or through liquid anesthetic contained in an “in-circuit” vaporizer. These diverted gases were returned to the mixture of fresh and rebreathed gas, ultimately delivered to the patient. Vapor concentration and anesthetic effect could not be predicted, and could produce excessive or inadequate concentrations. The Boyle’s vaporizer (bottle), invented by Henry Boyle (1875–1941), is an example of such a vaporizer. The glass with which it was made insulated the liquid anesthetic which thus would cool (although a water jacket surrounded the glass and limited the cooling). It provided a limited anesthetic surface area, and the control for the diversion of gases through the vaporizer was qualitative at best, indicating administration of more or less anesthetic, but not how much more or how much less. Other vaporizers, including one built by Victor Goldman (1903–1994), had some modern characteristics, but like many previous devices, they were usually designed for nonrebreathing use and did not provide for the quantitative delivery of anesthetic. Generations of anesthetists learned to cope with these deficiencies. I wanted something better.
In retrospect the desirability of producing precisely known concentrations of vapor for addition to the respired gas mixture is clear. My laboratory notebook contained several designs to achieve that end, but they all contained one notion – a separate measured flow of carrier gas, all of which bubbled through the vaporizer. In disguise, this principle continues to be applied in vaporizers used today (see below), long after the demise of the Copper Kettle. Oxygen was chosen as the carrier gas so that if the oxygen supply failed the addition of anesthetic vapor would cease (Fig. 53.4).
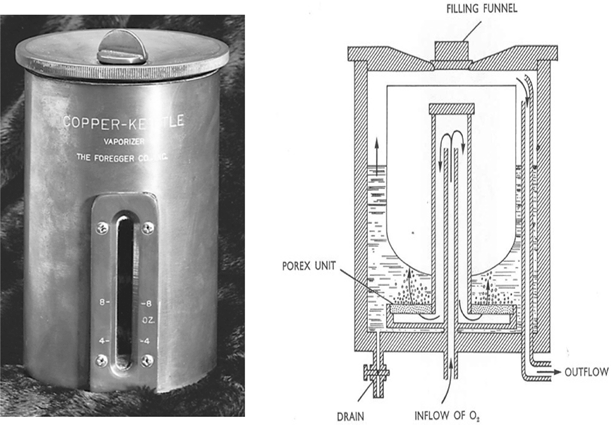
Fig. 53.4
The photograph of an early Copper Kettle on the left is from the ASA Newsletter 62:1998. The schematic on the right shows a precisely known flow of oxygen that enters through a diffuser leading to a porex filter that caused the gas to break into small bubbles, assuring that a maximum concentration of anesthetic was achieved in the gas, which then passed through an outflow tube, ultimately to be mixed with a second precisely known gas flow. Note the less safe filling funnel at the top of the vaporizer, a position that would permit the careless anesthetist to overfill the vaporizer and allow liquid anesthetic to enter the carrier gas stream, thereby potentially producing a lethal anesthetic concentration. Contrast this with the vaporizer shown in Fig. 53.7. (From the personal collection of the author)
The new vaporizer had two important additional components. First, to provide the heat necessary for vaporization, and thereby prevent undue chilling of the anesthetic, I replaced the nearly ubiquitous glass bottle of previous decades with a copper container (Figs. 53.4 and53.5). Copper was used because of its considerable capacity to conduct heat. I further increased temperature stability by attaching the copper container to a copper tabletop. Secondly, the metered gas was directed through a sintered bronze bubbler (see POREX in Fig. 53.4) at the bottom of the copper container with a resulting dispersion into a multitude of fine bubbles. This provided an enormous combined surface area, a large gas-liquid interface, one providing rapid saturation of the bubbling gas with anesthetic.

Full access? Get Clinical Tree







