59 Tachydysrhythmias
• Tachydysrhythmia may be due to intrinsic cardiologic disease or external stimulation.
• Noncardiologic causes, such as hypoxia, inadequate perfusion, metabolic derangements, or toxicity from medications or other agents, should always be considered.
• The higher the tachycardic heart rate, the greater the likelihood of instability.
• Patients with ventricular tachycardia may not necessarily appear unstable.
• If there is uncertainty, wide QRS complex tachycardia should be assumed to be ventricular in origin.
• The most effective treatment of ventricular tachycardia is electrical cardioversion. However, antidysrhythmic medications may also be necessary to prevent recurrence.
• All ostensibly healthy young patients with syncope should be assessed for valvular disease, left ventricular hypertrophy, Wolff-Parkinson-White syndrome, abnormal QT interval or T-wave morphology, and Brugada syndrome.
Epidemiology
Heart rhythms with rapid rates, or tachydysrhythmias, have a range of causes and associated incidences, morbidities, and mortality. Atrial fibrillation (AF) occurs in up to 5% of people older than 65 years. Conversely, sustained ventricular tachycardia (VT) is rare and accounts for less than 0.1% of emergency department (ED) visits. Morbidity ranges from temporary lightheadedness to syncope and deterioration, ventricular fibrillation (VF), or embolization and subsequent permanent disability as a result of ischemic stroke. Mortality from atrial tachydysrhythmias is generally low. Mortality from VT is approximately 5%, and that from VF ranges from 80% to greater than 90%, depending on the clinical circumstances.1 A particular exception to the low mortality associated with atrial tachydysrhythmias is AF or flutter with rapid ventricular excitation via a bypass tract, such as in Wolff-Parkinson-White (WPW) syndrome.2
Pathophysiology and Mechanisms
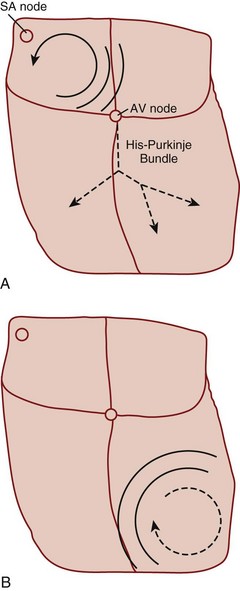
Reentry refers to a microscopic or macroscopic circular pattern of myocardial depolarization. A wave of excitation passes around the circuit and depolarizes the cells until it arrives at the beginning, and the process repeats itself. Reentry can occur if the circuit is sufficiently long or there is a slow-conducting portion that allows enough time for cells to have recovered from their refractory period before the wave of excitation returns (Fig. 59.1). The reentrant rhythm becomes the cardiac pacemaker if it is sufficiently fast to outpace the sinus node.
Presenting Signs and Symptoms
• The presence, periodicity, and morphology of the P waves
• The relationship of the P waves, if any, to the QRS complexes
• The morphology of the QRS complexes and other waves as compared with a previous tracing if available
Treatment
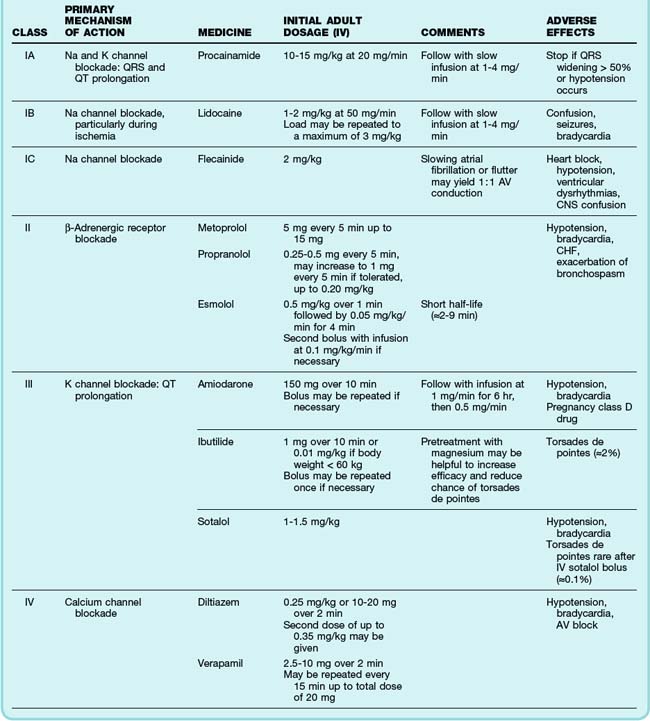
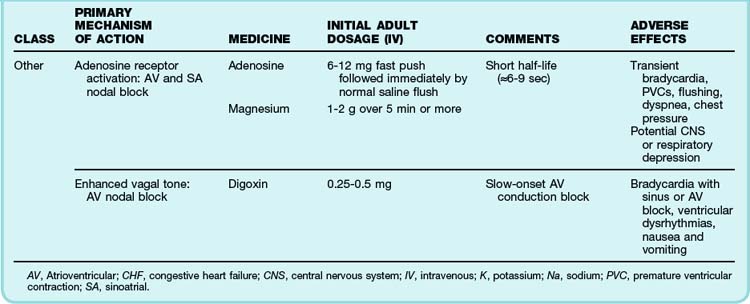
The primary emergency therapies for tachydysrhythmias include medications to slow electrical conduction or to increase the cellular refractory period in the AV node or other groups of cardiac cells (Table 59.1). The goal of therapy is to terminate and suppress the abnormal rhythm or to slow the response in the remainder of the heart. Electrical therapy, including synchronized cardioversion and defibrillation, is used in potentially or frankly unstable patients. Electrical therapy has the advantages of high efficacy and safety, but shocks are painful and do not prevent recurrence of the tachydysrhythmia. Patients with higher heart rates, regardless of the underlying rhythm, have compromised diastolic filling and cardiac output. These patients should be treated more aggressively with early electrical therapy. The emergency physician (EP) should understand and use antidysrhythmic medications but should minimize the use of multiple agents in a single patient. Use of multiple drugs increases the likelihood of drug interactions and compromised cardiac output.
See Table 59.1, Select Tachydysrhythmia Medications, online at www.expertconsult.com
Specific Tachycardias
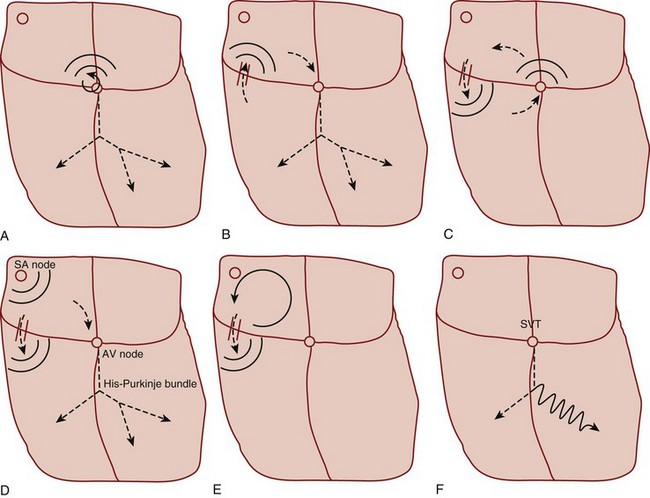
Primary tachycardiac conditions can be divided most simply into those of supraventricular and ventricular origin (Box 59.1; Fig. 59.2; see also Fig. 59.1). Supraventricular tachycardia (SVT) must involve tissue above the ventricles but may also involve ventricular tissue in a tachycardiac circuit, whereas VT involves only ventricular tissue below the AV node. The remainder of the chapter deals with the diagnosis and treatment of specific tachycardiac conditions.
Supraventricular Tachycardias
Irregular Supraventricular Tachycardias
Atrial Fibrillation
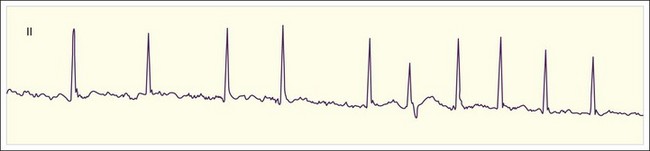
The clinician can usually diagnose AF at the bedside by feeling the pulse, listening to the heart sounds, and observing a single-lead cardiac monitor. The ECG may show fine or coarse irregular undulations of the baseline and an absence of regular P waves. The QRS complex is usually normal; however, if the ventricular response is rapid, the QRS complex may be transiently widened because of intermittent aberrant conduction through the His-Purkinje system (Fig. 59.3). This finding, termed the Ashman phenomenon, can occur when areas of the His-Purkinje tissue have a longer refractory period than the AV node does. Such bursts of wide QRS complex tachycardia can be confused with nonsustained VT, but on close inspection the rhythm remains irregularly irregular.
The most common treatment issue for patients with AF in the emergency setting is control of the ventricular rate. Depending on the function of the patient’s AV node, the ventricular rate may range up to approximately 150 beats/min in adults. Ventricular rates this fast allow insufficient time for diastolic filling of the heart. The rate can be slowed with a variety of agents that act to lengthen the refractory period of the AV nodal tissue, such as calcium channel blockers,3 beta-blockers,4 magnesium,5 digoxin, and amiodarone. Digoxin is less useful in the emergency setting because it takes a few hours to work, but the other agents must be used carefully because they can decrease blood pressure to varying degrees. The calcium channel blocker diltiazem usually provides excellent rate control with minimal loss of blood pressure. Beta-blockers may cause a greater loss of blood pressure and must be used with caution in patients with CHF or pulmonary disease. There is no indication for the administration of adenosine in patients with AF. The diagnosis should be made on the basis of findings from the history, physical examination, and ECG. Furthermore, adenosine, which only transiently blocks AV nodal conduction, would have no lasting therapeutic benefit.
Cardioversion can also be accomplished chemically when circumstances allow. Agents that slow conduction or increase the refractory period of atrial tissue are used to break the reentrant microcircuits within the atria. Vaughn-Williams class III and class I medications that can accomplish this goal include ibutilide, amiodarone, procainamide, propafenone, and flecainide.6,7 These agents can terminate AF alone and increase the likelihood of cardioversion of AF with subsequent DCCV. However, their use entails some risk. There is an approximately 2% to 5% chance of causing torsades de pointes (TdP) after the infusion of ibutilide, amiodarone, and procainamide because these agents variably increase the duration of the depolarized phase and the associated QT interval in the ventricles, as well as the atria. The newly developed agent vernakalant may be less likely to cause TdP because of its action in blocking the ultrarapid potassium channels located primarily in the atria. Hypokalemia and hypomagnesemia raise the risk for TdP. Pretreatment with magnesium may be protective in some circumstances, even in patients with normal serum electrolyte levels.8 Propafenone and flecainide can slow conduction, exacerbate heart failure, and cause ventricular dysrhythmias. Cardioversion also carries a risk for embolic stroke.
In the acute setting, clot may form within 48 hours after the onset of AF. Cardioversion of AF may cause a new clot to embolize into the systemic circulation. If the patient can clearly discern when he or she is in AF, symptoms have begun within the preceding 1 to 2 days, and the patient is not at high risk for stroke, it is generally safe to perform cardioversion without anticoagulation.9 However, if there is any uncertainty about the time of onset of the tachydysrhythmia or if it occurred more than 48 hours ago, the patient should not undergo immediate cardioversion. Instead, the cardiology service should be consulted and transesophageal echocardiography performed to search for atrial clot formation, or cardioversion should be deferred until adequate anticoagulation has been established.10 The only exception would be cardiovascular instability secondary to AF, which requires emergency DCCV. Continuation of anticoagulation should also be considered for 4 weeks after cardioversion because there may be some delay in return of normal atrial contraction.
The disposition of patients with AF depends on a number of factors, including age, comorbid illnesses and social and outpatient medical support, adequacy of control of the ventricular rate, plans for cardioversion, and the state of anticoagulation.11 If the patient requires ongoing adjustment of medications to control a rapid ventricular response, attempted cardioversion is planned, or new anticoagulation is initiated, hospitalization should be considered. The EP should make this decision in concert with the patient and the consulting cardiologist.
Multifocal Atrial Tachycardia
Multifocal AT (MFAT) is a relatively uncommon tachydysrhythmia that primarily affects older patients with chronic lung diseases such as chronic obstructive pulmonary disease, and it appears to be related to the administration of methylxanthine.12 Its mechanism is uncertain, but MFAT is thought to occur as a result of DADs in atrial tissue triggering ectopic beats. The diagnosis is made on the basis of the following ECG criteria: at least three consecutive P waves with different morphologies, variable P-P and PR intervals, and a heart rate greater than 100 beats/min. Treatment of MFAT is centered on treating the underlying pulmonary disease and possible hypoxia. Antidysrhythmic agents are poorly effective in slowing the atrial rate or the ventricular response or in terminating MFAT. Nevertheless, a carefully administered trial of a calcium channel blocker or amiodarone is reasonable. Generally, beta-blockers should be avoided in patients with any evidence of bronchospasm. Magnesium therapy, particularly in combination with potassium replacement, may slow the rate or terminate the tachydysrhythmia. Patients usually require admission to treat the pulmonary disease.
Regular Supraventricular Tachycardias
Atrial Flutter
Atrial flutter can occur as a result of a variety of cardiopulmonary and metabolic derangements, often in association with atrial dilation. Patients with atrial flutter also tend to experience AF, but atrial flutter is less common overall. The mechanism of atrial flutter is a macroreentrant circuit in the right atrium (see Fig. 59.1, A). The most common variant of atrial flutter is termed typical atrial flutter and involves a counterclockwise wave of roughly circular excitation when viewed from a position facing the front of the patient. The flutter pathway is constrained in the inferior portion of the circular loop by anatomic structures, and it travels between the tricuspid annulus anteriorly and the inferior vena cava and coronary sinus posteriorly. This segment of the pathway, the isthmus, contains the relatively slower-conducting tissue in the reentrant loop. Atypical atrial flutter most commonly travels over the same pathway, but in the opposite direction.
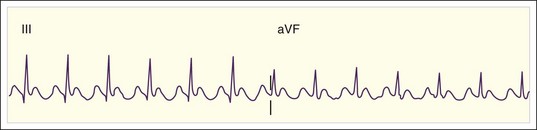
The rate of atrial flutter waves ranges from 250 to 350 beats/min. The diagnosis is made from the ECG tracing, which demonstrates typical sawtooth-shaped flutter waves at the appropriate rate, generally most prominent in the inferior limb leads (II, III, and aVF) and lead V1. The flutter waves tend to be identical and to recur regularly, and there is no isoelectric or flat ECG segment between the waves (Figs. 59.4 and 59.5). As in AF, the refractory period of the AV node usually controls the ventricular response to atrial flutter. The most common response is 2 : 1 AV conduction; however, higher, lower, or variable ratios of conduction are also possible. The ratio of conduction depends on the atrial flutter rate, the health of the AV node, and any modifying physiologic, metabolic, or pharmacologic factors. Because atrial flutter is most commonly manifested as flutter waves at 300 beats/min, 2 : 1 AV conduction, and a ventricular response rate of 150 beats/min, atrial flutter should be the first diagnosis considered in all patients with a regular tachycardia at 150 beats/min. It is important to remember, however, that pharmacologic agents can slow the rate of atrial flutter and decrease or increase AV nodal conduction, thereby altering the usual characteristics.
Cardioversion of atrial flutter is associated with thromboembolism, although the association is probably weaker than that with cardioversion of AF.10 For this reason, cardioversion of atrial flutter should probably not be undertaken in the ED unless the indication is an emergency because of cardiovascular instability, the combined duration of atrial flutter and AF is less than 48 hours, the patient has been adequately anticoagulated before arrival in the ED, or the patient is newly anticoagulated without evidence of atrial thrombi on transesophageal echocardiography. The decision to hospitalize patients with atrial flutter involves weighing issues similar to those described for AF.
Atrial Tachycardia
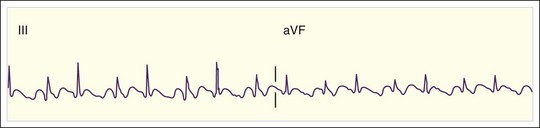
Regular AT is a relatively uncommon tachydysrhythmia. It may be due to cardiopulmonary disease with dilated atria and a reentrant mechanism; toxicity from digoxin, methylxanthines, or adrenergic agents with increased automaticity; or other causes. AT is distinguished from atrial flutter by separate identifiable P waves with an intervening isoelectric baseline and a rate lower than 200 beats/min (Fig. 59.6). Sometimes the P waves have an abnormal upward or rightward axis, or the onset of tachycardia is abrupt. These characteristics would distinguish the rhythm from sinus tachycardia. Finally, as discussed later, unlike reentrant atrial rhythms that involve the AV node, AT would not be expected to terminate with agents that slow AV nodal conduction. As with atrial flutter, decreasing AV nodal conduction dynamics might slow the ventricular response to the rhythm and facilitate the diagnosis. It would not generally terminate the rhythm because the origin of the tachycardia is contained entirely within the atria.