Chapter 70
Status Epilepticus 
Epidemiology
The most common causes of CSE are AED withdrawal or nonadherence to AED therapy in epileptic patients and cerebral vascular disease, whereas alcohol withdrawal, cancer, metabolic disorders, anoxia, poisoning, central nervous system (CNS) infections, and traumatic brain injury (TBI) also contribute. NCSE is likely 25% to 50% of the total incidence of SE although it has been as high as 81% in some series. In critical care patients, 75% to 92% of patients diagnosed with SE are found to be in NCSE as opposed to CSE. In a study of 570 ICU patients with altered mental status who were placed on continuous EEG monitoring (cEEG), 19% had NCSE. The increased availability and utilization of cEEG have undoubtedly increased the clinical team’s ability to identify NCSE in patients. The incidences for both CSE and NCSE are considerably higher in those older than 60 years of age.
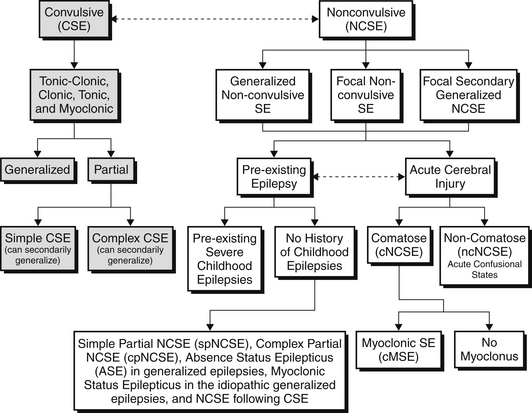
CSE by definition requires convulsive movements, defined as rhythmic movements or posturing of the extremities. These movements can be further classified into tonic-clonic, clonic, tonic, and myoclonic CSE. These subtypes are associated with specific EEG correlates at their onset. CSE can then be further subdivided into convulsions consisting of bilateral extremity movement (generalized) versus unilateral extremity or facial movement (partial/focal). Further differentiation is made depending on the patient’s level of awareness. In simple CSE, consciousness remains intact, whereas in complex CSE, consciousness is altered. Simple focal CSE and complex focal CSE may also have secondary generalization. If CSE is allowed to persist in any of these subtypes, it is possible that there will be attenuation of the motor symptoms and patients will transition into NCSE (Figure 70.E1).
NCSE in patients with acute cerebral injury can be further subdivided into patients who are comatose (cNCSE) or those who are noncomatose but in acute confusional states (ncNCSE). The comatose NCSE group includes a further subdivision differentiating those patients with cMSE or without myoclonus. All these subdivisions of NCSE can be divided into either generalized nonconvulsive SE, focal nonconvulsive SE, or focal secondarily generalized NCSE based on their clinical and electroencephalographic picture (see Figure 70.E1). Different classification symptoms do exist, and it should be noted that the term proper NCSE (pNCSE) denotes all patients who are in NCSE but are noncomatose and does not take into account the presence of acute cerebral injury.
Approximately 12% to 43% of SE cases fail to respond to first- and second-line therapies. The term refractory SE (RSE) is used commonly to describe SE that does not respond to initial dosing with benzodiazepines and at least one antiepileptic drug (Figure 70.1). Half of all patients with RSE have a prior history of epilepsy, but other causes are diverse and include hypoxic-ischemic injury, immune-mediated diseases, infections, toxic-metabolic syndromes, trauma, degenerative disorders, neoplasms, and endocrine disorders.
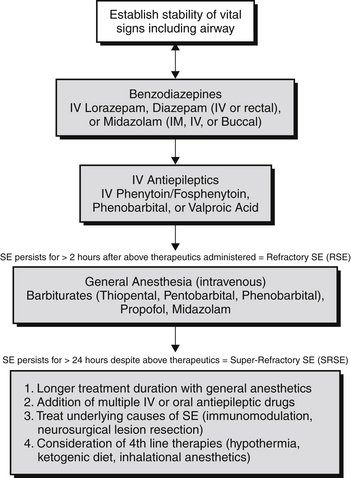
Figure 70.1 Treatment algorithm for status epilepticus. IV, intravenous; IM, intramuscular; SE, status epilepticus.
The term super-refractory SE (SRSE) is reserved for patients who continue to have seizures despite the use of general anesthetic agents (see Figure 70.1) to treat their SE or for whom seizures reoccur when therapy is tapered or withdrawn. SRSE may account for up 15% of SE cases. The most common cause of SRSE is acute severe brain injury, but other causes include immunologic, mitochondrial, infectious, toxic, and genetic disorders.
Pathophysiology
Seizures are the result of abnormal electrical discharges of cortical neurons and can arise because of abnormalities at any level in the central nervous system (CNS), from ions, receptors, cells, and networks, to the brain as a whole. Given that an overwhelming majority of seizures end spontaneously, it is hypothesized that an endogenous seizure terminating process must exist in the CNS or all seizures would be persistent. SE is attributed to a theoretic failure of the CNS’s innate ability to terminate an isolated seizure. This failure may occur through two mechanisms: persistence of excessive excitation or loss of normal inhibition. The mechanisms involved may include constant activation of the hippocampus, loss of inhibition by GABAergic interneurons on neurons in the hippocampus with intrinsic pacemaker capability, and increased glutamatergic excitatory synaptic transmission. All of these proposed mechanisms play a central role in epileptogenesis, leading to synchronous, repetitive firing of large populations of neurons. A genetic predisposition may also be involved in SE, shown by a higher incidence of SE in monozygotic than in dizygotic twins.