CHAPTER 69
Spinal Cord Stimulation: Hardware Specifications
INTRODUCTION
Spinal cord stimulation (SCS) offers hope to the estimated 75 million people who suffer from chronic pain. SCS sends electrical impulses that trigger nerve fibers selectively in the dorsal column based on the “gate control” theory. This interruption of pain signals traveling to the cerebral cortex causes feelings of pleasant and soothing tingling and paresthesia replacing the painful sensation of pain. Most commonly, SCS is utilized for the treatment of chronic neuropathic pain of the back, trunk, or extremities. Specifically these conditions include postlaminectomy syndrome, complex regional pain syndrome, diabetic neuropathy, as well as other off label uses have had great success with the SCS. SCS has successfully relieved pain in thousands of patients with severe chronic painful conditions.
Neuroaugmentation techniques have become increasingly popular in the specialty of interventional pain medicine due to its wide variety of on-label and off-label applications. Most products are manufactured by Medtronic, St. Jude Medical, and Boston Scientific, and have been undergoing continuous evolution. Competition and innovation in the industry has led to advanced technology allowing for variable programming and smaller sizes to make it physically acceptable to patients. For example, the old leads with 4 electrodes, also known as contacts, have been replaced by leads with 8 electrodes. Currently, predominantly cylindrical leads are used for percutaneous trials and paddle leads for laminectomy procedures. Now thinner paddle (surgical) leads, previously implanted exclusively with a laminotomy and surgery, are now being implanted percutaneously. Although the basic device works on the principles of the “gate control” theory, each company offers products with unique advantages.
The advantages of SCS therapy include:
• It is a nonpharmacologic option to control chronic pain.
• By avoiding medications, there are no psychosocial or medicolegal implications related to drug abuse, addiction, or tolerance.
• The system is reversible and can be removed without any harm to the patients, unlike spine surgeries that cannot be reversed.
• The SCS can be programmed based on the chronic pain severity and location of pain.
• Patient has ability to use multiple programs as desired for different situations, including fully turning a device off or on.
• As pain location, quality, or characteristics may change, there is an opportunity for regulation of electrodes to adapt to lead migration, or new location and quality of pains.
INDICATIONS FOR SCS
Chronic, intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with various conditions.
On-label indications are:
• Failed back syndrome (FBS) or low back syndrome or failed back
• Radicular pain syndrome or radiculopathies resulting in pain secondary to herniated disk
• Degenerative disk disease (DDD) refractory to conservative and surgical interventions
• Complex regional pain syndrome (CRPS) I or RSD
• Complex regional pain syndrome II or causalgia
• Epidural fibrosis
• Arachnoiditis
Off-label indications are:
• Extremity pain due to ischemia
• Angina resistant to other therapies
• Chronic headache not responding to other treatments
• Typical and atypical facial pain
• Postsurgical neuropathies (postherniorrhaphy, post-thoracotomy syndrome)
• Postherpetic neuralgia
• Abdominal visceral pain
• Pelvic visceral pain
• Vulvodynia
• Rectal pain
• Peripheral neuropathy (diabetic or metabolic)
• Peripheral entrapment neuropathy
THE COMPONENTS OF THE SCS DEVICE
The basic components of a spinal cord stimulator include:
• Leads
• Anchors
• Connecting extensions
• Implantable pulse generators (IPG) or battery
• Programmer
• Charger
Typically, epidural leads are anchored to lumbar fascia to minimize lead migration, and then connected to IPG directly or via connecting extensions. These pulse generators contain programmable elements of the system and specific battery life of the device. The IPG is either nonrechargeable (conventional) or chargeable. Nonrechargeable is also referred as primary cell battery. The implantable pulse generator is also referred as battery or neurostimulator.
The major components of the stimulator system will be discussed below with comparison of products of the 3 major companies: Boston Scientific, St. Jude Medical, and Medtronic including some of the indications, advantages, and disadvantages of each system.
Epiducer lead delivery system: It simplifies the multiple lead placements:
• Allows to percutaneously insert S-lead-paddle that normally would require laminectomy.
• Allows to configure patient-specific multiple lead arrays.
SCS Leads
Depending on whether the physician is implanting the SCS leads for a trial temporarily or permanently, the terminology referring to the lead is as a “trial lead” or a “permanent lead.” However, both leads are essentially the similar product. The selection of leads depends on source of pain, complexity of pain generators, physician preference and ability, and comorbidities.
Percutaneous or Cylindrical Leads
Overview
• These are cylindrical insulated catheters with multiple sequential electrodes with variable spaces mounted near the distal end.
• Used as trial or permanent leads.
• The octrodes have 8 electrodes and quads have 4 electrodes mounted.
• The leads may vary in length, number of electrodes, spacing between contacts and diameter.
• Leads can be implanted through a needle and may not need a large surgical incision.
• The percutaneous leads are faster and easier to place.
• They are more prone to migration than paddle leads, which are placed surgically.
• The cylindrical electrodes are less energy efficient due to circumferential energy dispersion.
• 360-degree stimulation of cylindrical leads can lead to painful areas of stimulation along the posterior epidural space.
Due to increased number of electrodes in one lead, the programming of electrode configurations and ability to steer stimulation have expanded.
Spacing of the percutaneous leads: 4 or 8 individually current controlled contacts per lead spaced apart depending on the number of contacts on a single lead. Usually, for a quad lead the spacing is 7 to 9 mm and for octad 4 to 5 mm allowing twice the contacts within the same “quad” spacing for precise targeting by a single lead (Figure 69-1).
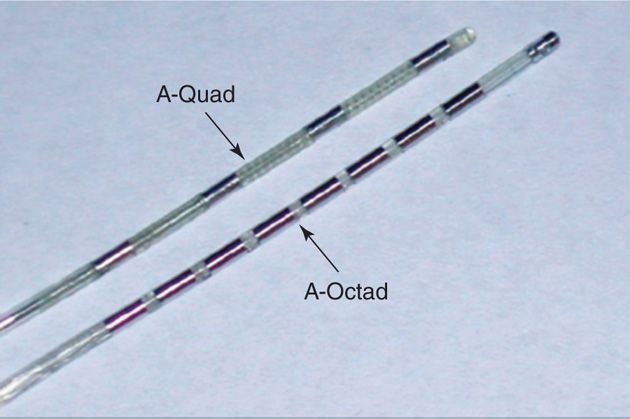
Figure 69-1. Contacts spacing. A, quad lead with 9-mm-wide spacing; B, octad with 4-mm compact spacing. (Image courtesy of Boston Scientific Corporation.)
The octad leads:
• A percutaneous octad lead with 8 contacts is used for single and dual lead placement and configuration.
• Octad leads cover 2 or more vertebral segments, helping to optimize pain coverage.
• They provide electronic “repositioning” to manage changes in stimulation coverage due to vertical lead movement.
• They provide the longest contact span and most spacing options.
• Percutaneous leads may be configured to create simple or complex contact arrays, utilizing up to 2 leads for one IPG.
Surgical or Paddle Leads
Overview
• Wide variety of designs, that provides multiple options.
• Paddle leads are flat, with insulated back and sides force current to flow anteriorly toward the dorsal column.
• These leads are larger, and require a surgical incision as well as a laminotomy for placement, due to the larger size and shape.
• A single column paddle lead can be placed percutaneously without a laminotomy by a specifically designed introducer called the Epiducer lead delivery system.
• The surgical leads are less prone to migration than percutaneous leads.
• The flat shape with insulated back and sides makes them more energy efficient due to unidirectional energy penetration, leading to longer battery life.
• The placement of leads requires a surgical procedure, and often requiring a laminotomy.
• It is not possible to trial a surgical lead percutaneously.
• Often, paddle leads are used for more complicated pain patterns and spinal anatomy.
Surgical leads are typically used for permanent lead placement, but the exception to the rule may be when difficulty encountered accessing the epidural space.
Surgical Leads
1. Single-column paddle leads
• These leads offer benefits of paddle leads in a steerable, low profile design with extensive lead configuration and placement options.
• Narrowest paddle available can be placed through a small laminotomy procedure, which is steerable and provides easier insertion and reduced trauma to soft tissue and bony structures.
• Flexible, styleted paddle tip is designed to enhance steering and control.
• Insulation on 3 sides to provide an efficient, unidirectional electrical field, which focuses the stimulation on the neural target and decreases power requirements.
• Can be combined for multiple lead configurations.
2. Two-column paddle leads
• Insulated on 3 sides to provide unidirectional stimulation for enhanced precision in energy delivery and greater system longevity. Can be configured with other paddle leads to manage complex pain patterns.
• Narrow leads and curved designs help facilitate midline placement and stability.
3. Three-column paddle leads
• Transverse tripolar array designed to overcome anatomical challenges and provide control in shaping the stimulation field.
• Three columns of electrodes provide anodal blocking to confine stimulation to the midline and can be used for hard-to-cover back pain, may create transverse tripolar array.
• Narrow leads for smaller anatomy and placement flexibility. Anatomically curved designs help facilitate midline placement and stability.
4. Five-column (penta) paddle leads
• Smallest contacts in a five-column array for precise field control and broad lateral coverage.
• Designed to enable selective nerve fiber stimulation and predictable dermatomal activation, it provides enhanced control for coverage of complex, multifocal pain. Broad lateral contact span designed to accommodate anatomical asymmetries as well as placement variability.
• Small contact size designed to focus current for enhanced specificity.
• Wide-spaced leads and compact-spaced leads.
Selection of Leads
Each lead, whether cylindrical or paddle, has at least 4 contacts, but a lead can contain as many as 16 contacts. The number of contacts used depends on the condition being treated as well as the physician’s preference. For example, more complex pain patterns, such as those involving more than one area and more than one extremity (arms and/or legs), involve more nerve structures will require two octad leads. Thus, additional leads with more contacts may often be required to stimulate all of these structures.
Implanting a single lead with fewer contacts than you need may result in less pain relief. In fact, many physicians believe that it is best to implant extra contacts to make up for the unexpected change in the intensity and location of, and for potential migration of lead/leads. If that occurs, pain relief is achieved by “electronically repositioning” the contacts. Electronic repositioning is accomplished by reprogramming the stimulation parameters, power, pulse width and frequency, which requires a visit to the physician’s office.
Connecting Extensions
• Connecting extensions are used for larger patients where the length of the leads is short to reach the IPG, and to provide slack to minimize the potential for lead migration.
• This extra piece of equipment can be a source for mechanical error if there is high impedance and poor pain relief for a patient.
Anchors
These are small devices used to anchor the leads to reduce lead migration and breakage. Ideally, it should be radiopaque allowing easy identification under fluoroscopy.
• Should be easy for the placement, and easily accessible in the incision to minimize lead migration and provide security to the epidural leads.
• The locking device should lock the anchor to the lead, and unlock if repositioning of the lead or the anchor is necessary. It should be visible under fluoroscope.
1. Boston Scientific Clik anchor (Figure 69-2)

Figure 69-2. Boston Scientific Clik anchor. (Image courtesy of Boston Scientific Corporation.)
• Locking system uses a turn of a hex wrench, providing tactile and audible confirmation that lead is secure.
2. Medtronic anchors
• Titan anchor
• Titanium body with silicon anchor
• Further adhesive bond utilized
• Bumpy anchor
• Bi-Wing anchor
• ADT Bi-Wing anchor and lead unit
3. St. Jude Medical Cinch™ anchor
• Titanium retention sleeve provides a holding force higher than conventional silicone anchors.
• Extended distal strain relief reinforces the lead where it exits the vertebral column to reduce lead fatigue and subsequent lead fracture.
• Radio-opacity facilitates identification under fluoroscopy.
4. St. Jude Medical Swift-Lock anchor
• Mechanical locking anchor requires a 90-degree twist to lock into place.
• The anchor can be disengaged and repositioned on the lead if necessary.
• Eliminates the need for sutures or medical adhesive to fasten the anchor to the lead.
• Extended distal strain relief reinforces the lead where it exits.
IMPLANTABLE PULSE GENERATORS
The implantable pulse generator (IPG), also called neurostimulator, is a battery powered device which generates electrical pulses and delivers stimulation through one or more leads. The IPG system is implanted and connected to leads directly or via connecting extensions, sending signals from the device to the spinal cord via the electrical leads. Certain companies store the patient’s programs into the battery as well.
Type of Stimulation Based on Energy Source
In the delivery of electrical energy to biological tissues, stimulus devices can hold either current or voltage constant during the stimulation process of maximum 16 contacts.
• Voltage constant system. Devices that hold voltage constant at a value set by the user and allow current to be determined by Ohm’s law are known as “constant voltage stimulators.” Medtronic uses constant voltage.
• Current constant system. Devices that hold current constant at a value set by the user during the stimulation process and allow voltage to be determined by Ohm’s law are called “constant current stimulators.” St. Jude Medical has a single source current constant IPG that controls all 16 contacts.
• Independent energy source. With 10 mA on entire lead, the amplitude on each contact is individually specified and maintained even when impedances change. BSC has the independent current system.
The system provides 3 basic parameters:
1. Pulse width
2. Frequency
3. Amplitude
A program is a specific combination of pulse width, rate, and amplitude settings acting on electrode combination up to 16 electrodes per program. Up to 4 programs can be combined into a group. When using more than 1 program, the pulses are delivered sequentially—first a pulse from one program, then a pulse from next program. Pulse width, amplitude, and electrode polarity of each program within the group can have different values.
Types of IPGs include:
• Non-rechargeable (Conventional SCS Technologies)
• Rechargeable (Advanced SCS Technologies)
• External pulse generators
The IPG devices are used to treat chronic, intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following conditions:
• Failed back surgery syndrome (FBSS) or low back pain syndrome
• Radicular pain syndrome or radiculopathies resulting in pain secondary herniated disk
• Postlaminectomy pain
• Degenerative disk disease (DDD) and herniated disk pain refractory to conservative and surgical interventions
• Peripheral causalgia
• Epidural fibrosis
• Arachnoiditis or lumbar adhesive arachnoiditis
• Complex regional pain syndrome I (CRPS I) or reflex sympathetic dystrophy (RSD)
• Complex regional pain syndrome II (CRPS II) or causalgia
CONVENTIONAL NON-RECHARGEABLE PULSE GENERATORS
Advantages
• Simpler to use for the patient
• Cheaper
• Fully implantable
• Primary cell battery
• Nonissue with patient compliance
Disadvantages
• Require replacement in 5 to 6 years, although variable based on usage
• May result in short life span and repeat replacement surgeries
• Radiofrequency IPG requires patient to wear external equipment to power the implant
RECHARGEABLE PULSE GENERATORS
Advantages
• Longer life span (10-12 years)
• Smaller IPG
• Reduce the need for surgical procedures due to less frequent replacement, saving expenses for the surgical procedure and potential complications
• Increased stimulation parameters and therapy options
• Suitable for patients with high energy demands
• Supports complex programming
Disadvantages
• Complexity of recharging
• Inconvenience
• Initial higher cost
• Potential lack of compliance
Rechargeable IPG by Boston Scientific (Figure 69-3)
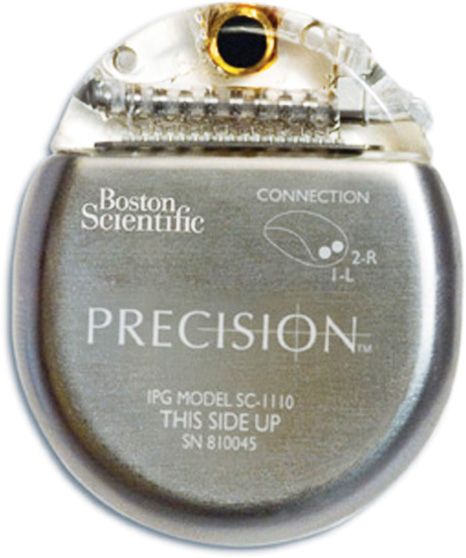
Figure 69-3. Precision Plus rechargeable IPG. (Image courtesy of Boston Scientific Corporation.)
• Cordless patient remote control and charger with a wireless range of up to 24 in.
• Precisely targets pain by moving the electric field between simultaneously active contacts in 1% increments with 1 mm spaced contacts to minimize dead spots and sculpt the electrical field.
• May automatically adjust impedance changes to maintain therapy when leads scar in.
• Oval shape that may provide patient comfort.
• This company do not produce primary cell non-rechargeable IPG.
Bion Microstimulator by Boston Scientific (Figure 69-4)

Figure 69-4. Bion Microstimulator by Boston Scientific. (Image courtesy of Boston Scientific Corporation.)
• It is a rechargeable battery powered electrode.
• Implanted near the occipital nerve for chronic HA.
• It is controlled by wireless remote control.
IPGs by Medtronic
Non-rechargeable conventional IPG (also called primary cell neurostimulator):
1. RestorePrime stimulator model 37701
2. PrimeAdvanced neurostimulator model 37702
3. Itrel 3 neurostimulator model 7425
• 39 cc, 16-electrode neurostimulator with ability to shape a stimulation field between 1- and 4-lead arrays, which can be steered in any direction.
• Multi-programmable device may store up to 4 programs in a specific combination of pulse width, rate, and amplitude settings acting on a specific electrode combination, up to 16 electrodes per program.
• When using more than one program, the pulses are delivered sequentially, ie, first a pulse from one program, then a pulse from the next program.
• Option for patient to self-select parameters, enhancing comfort and self-control, minimizing office visits.
• Pulse width, amplitude, and electrode polarity for each program within the group can have different values.
• Rate, rate limits, ramping, and cycling for each program within the group have the same values.
Rechargeable Medtronic IPG
1. RestoreUltra neurostimulator model 37712
2. RestoreAdvanced neurostimulator model 37713
3. RestoreSensor neurostimulator model 37714
• Multi-programmable, rechargeable device which delivers stimulation through 1 or more leads in the epidural space
• Thin, small, 22-cc, 16-electrode device with up to 4 leads with steering capacity
• May create between 1- and 4-lead arrays with patient controlled parameters
Restore Advanced Rechargeable Battery
• Multi-programmable rechargeable neurostimulator
• It listens and senses the changes in the patient position.
• It automatically adjusts and reprograms to optimize the settings per change in position.
• It has ability to record patient’s objective data.
St. Jude Medical IPGs
1. The EonC non-rechargeable IPG.
• Ideal choice for patients with low to medium power requirements and those who prefer the simplicity of a non-rechargeable IPG.
• Wide range of pulse widths, frequencies, amplitudes which adjust to impedance and treat complex pain patterns.
• Features greatest battery capacity of any primary cell IPG battery that does not require recharging, making it less complex, and easier to maintain. The longevity of a primary cell IPG depends on the energy requirements of the patient. At certain settings, small primary cell IPGs will last more than 5 years and high-capacity primary cell IPGs can last 10 years or longer.
• Compatible with a comprehensive line of neuromodulation leads and extensions for easy replacement of depleted competitive IPGs.
• Primary cell IPGs are available in 8 or 16 contact constant current, primary cell IPG that automatically adjusts power output to maintain prescribed stimulation as impedance levels change; patient may control up to 8 pain areas at one time.
2. The Genesis non-rechargeable IPG
• Eight-contact primary cell IPG system
• Compact size with high battery capacity among small primary cell IPGs
• Efficient circuitry and high-capacity battery
• Suitable for stable unilateral extremity pain
• Limited power requirements
• Suitable for elderly patients or patients with limited cognitive ability
• Anxious about technology
• Life span 2 to 6 years
3. Rechargeable Eon Mini IPG
• NeuroDynamix technology enables longer battery life and time between fast recharges, expected greater than 24 hours between recharges at high settings, with a 10-year life expectancy at high settings.
• FDA approved for last 10 years at high stimulation settings, which means patients may require fewer replacement surgeries, and it is designed to provide consistent pain therapy for virtually any pain profile. If lower parameters are used, a significantly longer device life is possible.
• Smallest 18 cc IPG with a deeper implant depth of up to 2.5 cm to enhance patient comfort and placement options, less tissue erosion, and without compromising recharge efficiency.
• Dynamic MultiStim programming and 16 contact header compatibility allows control of up to 8 pain areas simultaneously.
• Offers both MultiStim and PC-Stim (patient-controlled stimulation) capabilities, giving clinicians and patients control of the stimulation therapy and their individual pain pattern treatments—multifocal pain pattern.
• High power requirements.
• Patient must have cognitive ability to grasp recharging responsibility and burden.
• May not be suitable for elderly patients.
4. External radiofrequency Renew IPG
St. Jude Medical is the only company that provides a pulse generator with an external battery source. The pulse generator is actually a receiver that receives energy by radiofrequency from external source. This may be an alternative option specifically for peripheral nerve field stimulation.
• The Renew system consists of a radiofrequency transmitter and an external receiver for percutaneous leads or paddle leads.
• Initial high frequency high power technology
• Powerful 8- or 16-contact systems with broad range of power outputs
• Renew radiofrequency (RF) systems are designed to sustain therapy over long periods at high output levels. Frequency ranges up to 1500 Hz.
• Does not need to be recharged, no replacement surgery required for life time.
• Small size, may be useful for peripheral nerve field stimulation.
• Receiver internalized and the transmitter is worn externally.
• Potential skin reaction from external component
• This system is not used frequently.
THE SCS EXTERNAL TRIALING SYSTEM
The external neurostimulation trialing system is used to evaluate spinal cord stimulation during lead placement or test stimulation. The addition of the multi-lead trialing cable to the external trialing system provides secure, confident trialing of more than one lead with less equipment necessary. Although this portion of the trial is often managed and supported by the representatives and technicians from the device company being used, it is important to understand the components, process, and technology.
External trialing system components include:
• Multi-lead trialing cables
• External trialing neurostimulator
• Test leads
• Clinician programmer
• Patient programmer
The external trialing system should provide the following benefits:
• Small, thin, curved anatomical design to provide comfort during trialing.
• To individually trial leads, it should provide a multi-lead trialing cable to support any program combination of quad or octad leads, up to 16 electrodes.
• Should be single cable and no extensions.
• The cable connections with trial leads and securing to skin externally should be easy, comfortable, and user friendly.
The external neurostimulator and the implantable neurostimulator produce comparable symptom suppression when set to the same parameter settings.
RECHARGING THE IPG
The primary cell conventional non-rechargeable IPG will require a simple surgery to get the battery changed at the end of life of battery. However, if a rechargeable battery is used, periodically it will need to be recharged via the implanted device. The amount of time it takes to charge the generator varies according to the brand and model, and age and condition of the device (about 10 years). The same is true for the amount of time the battery can hold a charge, ie, how long between charges. The older the battery, the more time charging takes, and the more often you will need to recharge.
• To charge a neurostimulator battery, the charger is held over the area where the device is implanted for a specified period of time.
• Traditional charges often either required a cord connected to a device that lies over the IPG site, however, now, there are cordless devices that can be utilized.
• Similar to cell phones, conventional SCS systems rely on rechargeable batteries that fail or suffer irreversible damage when over-discharged.
The available chargers are:
• Boston Scientific Precision Plus system charger powered by a zero-volt battery (Figure 69-5)
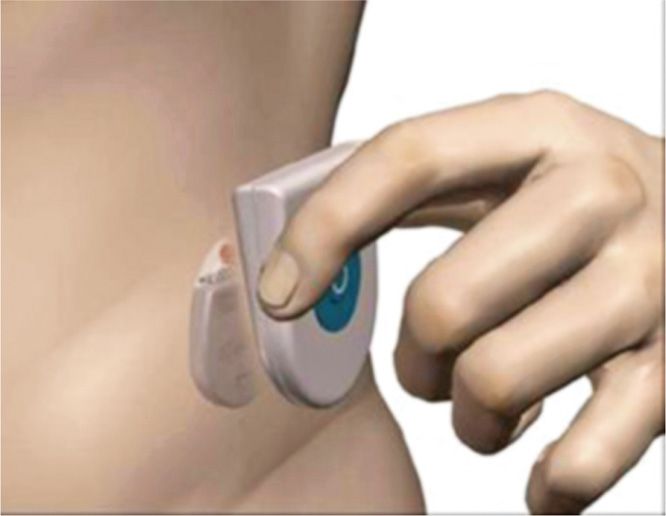
Figure 69-5. Boston Scientific Precision Plus system cordless charger. (Image courtesy of Boston Scientific Corporation.)
• St. Jude Medical Eon Mini charging system for smaller and longer lasting rechargeable battery that gives 10 years of anticipated life span
• Medtronic RestoreAdvanced and RestoreUltra chargers
SCS PROGRAMMERS
SCS programming involves selecting and optimizing the 4 variables for configuration:
• Stimulating electrode configuration
• Amplitude (current/voltage)
• Pulse width
• Frequency of electrical pulses
Amplitude (current/voltage) indicates the intensity of stimulation and paresthesia. This is set within a range of 0 to 10 V according the type of electrode used and the type of nerves stimulated. Increasing the voltage increases the area of capture in the spinal cord by recruiting nerves farther from the contacts. However, increasing the voltage can produce discomfort. Lower voltage is chosen for peripheral nerves and paddle type electrodes. Ideally paresthesia should be felt between 2 and 4 V.
Pulse width refers to the duration of a single stimulation pulse and usually varies from 100 to 1200 μs. Widening the pulse width will also broaden the area of paresthesia. By SCS blocking a stimulus, depolarization and propagation of an action potential of the nerve is prevented, avoiding the pain response. Lower amplitudes of stimulation at wider pulse widths are better tolerated by patients.
The frequency of the pulse wave is usually between 20 and 120 Hz. It is an individual preference: some patients choose low frequency beating sensation (low back and leg pain) whereas others prefer high frequency buzzing (CRPS).
Selection of lowest possible setting on all parameters is important in conserving battery life of SCS. Cycling of stimulation is also employed to save battery life. Changing the stimulator’s program may occur during the course of therapy and follow-up as the patient heals, leads scar over, or the nature of the patient’s pain changes.
Boston Scientific Programmer (Figure 69-6)
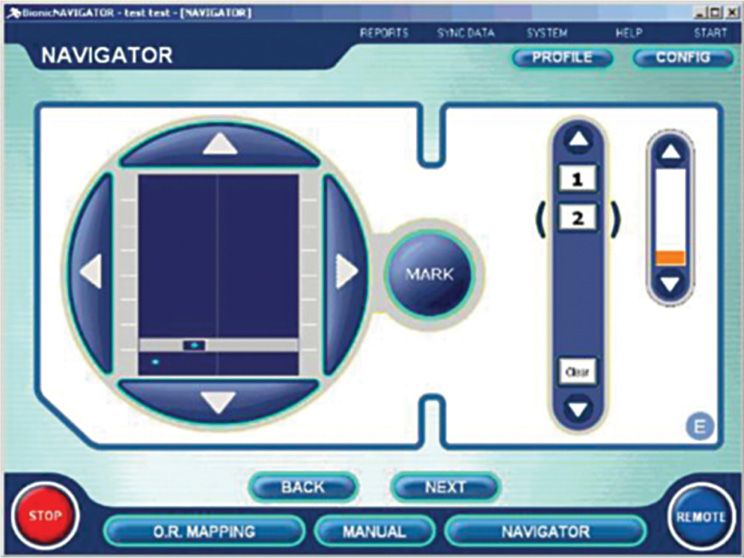
Figure 69-6. Boston Scientific Bionic Navigator. (Image courtesy of Boston Scientific Corporation.)
• Continuous real-time adjustment of stimulation settings allows patient to steer field to cover pain areas.
• 15-minute using navigation allows evaluation of hundreds of stimulation configurations.
• A collaborative approach to patient fitting.
• Patient assumes a greater level of control over their pain coverage.
Medtronic Programmer
Medtronic N’Vision Clinician Programmer. The Medtronic N’Vision model 8840 clinician programmer
• It is a portable device that offers a single programming platform for Medtronic implantable drug delivery and neuromodulation devices.
• It programs the RestoreSensor, RestoreUltra, Restore Advanced, RestorePrime, and PrimeAdvanced spinal cord neurostimulators, and SynchroMed II intrathecal pump.
• The MyStim model 37744 is a patient programmer.
• It is a handheld device that increases a patient’s ability to manage his or her own therapy, within clinician-set parameters, while receiving spinal cord stimulation.
St. Jude Medical Clinician Programmer
Designed to capture complex, multifocal pain, the St. Jude Medical Rapid Programmer 3.0 system with multisteering technology efficiently locates desired stimulation areas, assesses their interaction in real time, and fine-tunes settings for optimal coverage. It also simplifies the programming of multifocal pain to help deliver optimal therapy and meet each patient’s need.
• Provides the ability to steer current in one area while another remains active, “multisteering,” facilitating the programming of multifocal pain.
• Enables a comprehensive evaluation of stimulation patterns to enhance the likelihood of successful outcomes.
• Designed to produce a patient-perceived change in stimulation with each incremental step used to steer current.
St. Jude Medical PC-Stim Programming
• Allows multiple programs to be stored in the patient programmer manually selected by the patient.
• PC-Stim programming enables the patient to self-regulate therapy according to their pain level, body position, or activity level, for up to 24 programs.
St. Jude Medical Active Balancing Programming
• Active balancing programming allows fine-tune stimulation levels in multiple coverage areas concurrently to improve patient’s comfort level.
• Streamlined programming can adjust levels in multiple coverage areas simultaneously to quickly establish stimulation comfort levels.
• Intelligent amplitude control allows the system to synchronize multiple areas of coverage and enables patients to change individual amplitudes with a master control.
St. Jude Medical Dynamic MultiStim Technology
• Rapid Programmer 3.0 is built in such that 8 independent arrays can be blended for complex pain patterns.
• Constant current delivery and simple electrode arrays improve accurate steering of current to targeted electrodes even when impedance changes over time.
• Real-time programming allows patients to evaluate stimulation levels to multiple coverage areas as programming adjustments are made.
SCS CONTRAINDICATIONS
As per current United States FDA guidelines, the SCS system is contraindicated for patients with demand-type cardiac pacemakers, although patients in Europe have safely implanted stimulators in cardiac patients with good efficacy as reported in case reports. Thus, specifically in the US, SCS devices are avoided in patients with pacemakers.
In addition, patients who are unable to operate the system, have not passed an appropriate psychological evaluation for use of SCS, or failed to receive effective pain relief during trial stimulation should not be implanted with a neurostimulation system.
Important Warnings in Regards to SCS
• Neurostimulation should not be used on patients who are poor surgical risks or patients with multiple illnesses or active general infections.
• Electrocautery. Do not use short-wave diathermy, microwave diathermy, or therapeutic ultrasound diathermy on patients implanted with a neurostimulation system. Energy from diathermy can be transferred through the implanted system and cause tissue damage at the location of the implanted electrodes, resulting in severe injury or death.
• Cardioverter defibrillators. Neurostimulation systems may adversely affect the programming of implanted cardioverter defibrillators, although in Europe case reports have reported safe concomitant use.
• Magnetic resonance imaging (MRI). Patients with implanted neurostimulation systems should not be subjected to MRI. The electromagnetic field generated by an MRI may forcefully dislodge implanted components, damage the device electronics, and induce voltage through the lead that could jolt or shock the patient.
• Pregnant patients. Safety and effectiveness of neurostimulation for use during pregnancy and nursing have not been established.
• Implantation of two systems. If 2 systems are implanted, ensure that at least 20 cm (8 in) separates the implanted IPGs to minimize the possibility of interference during programming.
• Implantation of multiple leads. If multiple leads are implanted, leads and extensions should be routed in close proximity. Nonadjacent leads can possibly create a conduit for stray electromagnetic energy that could cause the patient unwanted stimulation.
• Postural changes. Changes in posture or abrupt movements may result in a decrease or increase in the perceived level of stimulation. Perception of higher levels of stimulation has been described by some patients as uncomfortable, painful, or jolting. Patients should be advised to turn down the amplitude or turn off the IPG before making extreme posture.
• The metal screening devices. Certain devices used at entrances or exits of public establishments, and airport security screening devices may affect stimulation. It is recommended that patients use caution when approaching such a device and that they request assistance to bypass the device. If they must proceed through the device, patients should turn off the IPG.
• Cellular phones. The effect of cellular phones on neurostimulation systems is unknown; patients should avoid placing cellular phones directly over the system.
• High output ultrasonic and lithotripsy. The use of high output devices, such as an electrohydraulic lithotripter, may cause damage to the electronic circuitry of an implanted IPG. If lithotripsy must be used, do not focus the energy near the IPG.
• Therapeutic radiation. Therapeutic radiation may damage the electronic circuitry of an implanted neurostimulation system. Sources of therapeutic radiation include therapeutic x-rays, cobalt machines, and linear accelerators. If radiation therapy is required, the area over the implanted IPG should be shielded with lead.
Availability of multiple electrodes and multiple leads has made possible to cover complex pain patterns by multiple programming capabilities. Devices are available to insert paddle leads percutaneously. Smaller IPG devices have led to greater comfort, and rechargeable devices have changed the reoperation rates and overall lasting nature of these devices. Perhaps with expansion into MRI compatibility, as well as a more expansive treatment options with increased on-label and off-label indications, including ischemic and neuropathic therapies, we look forward to continued collaboration of industries and health care providers for newer generations of these devices and further developments in interventional techniques to help our patients.

Full access? Get Clinical Tree







