Chapter 101 Shock and cardiac disease in children
Most cases of shock in childhood are caused by hypovolaemia (Table 101.1) or sepsis. The causes, clinical course, management and complications of shock differ from those in adults: severe diarrhoeal disease and congenital abnormalities are common in childhood but abdominal sepsis, pancreatitis and obstructive vascular disease are uncommon.
Table 101.1 Causes of hypovolaemic shock in childhood
| Water deprivation (absolute or relative to losses) |
The following factors affect the epidemiology of shock in children:
SMALLER BODY FLUID COMPARTMENTS
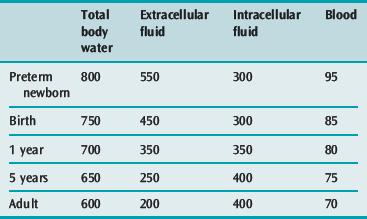
A small volume of blood or diarrhoeal fluid loss, or of fluid leakage into the extravascular space may represent a large percentage loss of blood or extracellular fluid (ECF) volume in a small child (Table 101.2).
IMMATURE IMMUNE SYSTEM IN INFANTS AND TODDLERS2
Antibody response to bacterial polysaccharide capsular antigens (e.g. Streptococcus pneumoniae, Haemophilus spp. and Neisseria meningitidis) remains poor in the preschool years. Low IgG concentrations, immunological naivety and reduced natural killer (NK) cell activity predispose young children to frequent viral infections, and the newborn to severe herpes simplex infection.3
Reduced cytokine production, especially in the immunologically naive, may alter the features and duration of the shock syndrome, including the ability to develop a fever, and causes deficiency in most T-cell functions, including cytotoxicity and augmentation of B-cell differentiation.4
PATHOPHYSIOLOGY
The pathophysiology of shock is described in Chapter 11 which should be read in conjunction with this chapter. The following aspects of a child’s physiology affect the response to insults.
IMMATURE CARDIOVASCULAR SYSTEM5,6
LESS DIASTOLIC COMPLIANCE THAN THE ADULT HEART
MORE TOLERANT OF HYPOXAEMIA
Although the infant heart has fewer mitochondria per gram of tissue, and a lower oxidative capacity, it has a lower oxygen demand per gram, larger glycogen reserves and a higher capacity for anaerobic glycolysis.7 Glucose is the major myocardial energy source (rather than long chain fatty acids as in older children) and hypoglycaemia causes severe myocardial depression.
IMMATURE AUTONOMIC INNERVATION
The infant myocardium has fewer β-receptors, more α-receptors, and is more sensitive to noradrenaline (norepinephrine) than the adult. Stimulation of myocardial α-receptors in the infant is more likely to cause tachycardia than bradycardia.8
HIGH PULMONARY VASCULAR RESISTANCE8
At birth, after the first breaths are taken, the smooth muscle cells gradually relax and become more fusiform in shape. Pulmonary vascular resistance falls immediately, then gradually declines to adult levels by 6–8 weeks of age, and smooth muscle bulk falls to adult levels by 3–4 months.9 In the presence of lung disease, high pulmonary venous pressures (e.g. due to aortic stenosis or stenosis of the pulmonary veins) or large left-to-right shunts (e.g. ventricular septal defect (VSD) or truncus arteriosus), the high pulmonary vascular resistance and the extensive and bulky vascular smooth muscle fail to decline normally. The infant is then vulnerable to episodes of acute right heart failure whenever the pulmonary vascular resistance increases abruptly.
CLINICAL PRESENTATION OF SHOCK IN CHILDHOOD
HYPOVOLAEMIC SHOCK (SEE Table 101.1)
The child may have a history of fluid or blood loss, reduced fluid intake or bowel-related illness. Signs of dehydration (see Chapter 99), external bleeding or haematoma may be present. The presence of hypovolaemic shock implies a blood volume deficit greater than 30 ml/kg.
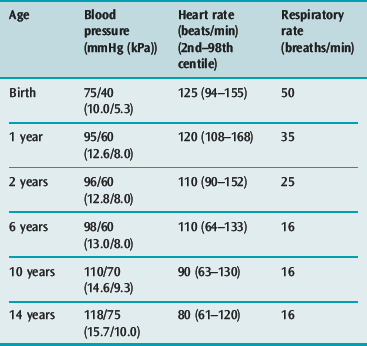
Signs of homeostatic compensation, such as tachycardia (Table 101.3), narrow pulse pressure, cool mottled limbs and slow capillary refill usually precede the onset of hypotension, which tends to occur late (after loss of 15–20% of blood volume) and precipitously in young children. In severe shock of any cause, signs of multiple organ hypoperfusion (e.g. oliguria of less than 0.5 ml/kg per hour, lethargy or coma, hypothermia, tachypnoea and increasing lactic or other metabolic acidosis) are found. Bleeding due to disseminated intravascular coagulation and liver dysfunction may occur in the first 6 hours.
Investigations (see Table 101.4) include:
Table 101.4 Investigation of shock in children
| All shocked children |
| If the cause of shock is unknown |
CSF, cerebrospinal fluid; ECG, electrocardiogram; PCR, polymerase chain reaction.
CARDIOGENIC SHOCK
Combinations of signs specific to individual congenital heart lesions are described below. Such signs when present (e.g. absent femoral pulses in aortic coarctation, or skull, liver or renal bruits of arteriovenous fistulae) may indicate the cause of the shock (Table 101.5), while diagnostically useful murmurs are not always present.
Table 101.5 Causes of cardiogenic shock in childhood
Investigations (see Table 101.4) should include:
SEPTIC SHOCK
Severely septic children are often hypothermic rather than febrile.
As shock progresses, myocardial depression by endotoxin, tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (see Chapter 11) decreases the cardiac output earlier than is the case in adults, as the less compliant infant ventricle cannot dilate to sustain the stroke volume in the face of a reduced ejection fraction.11,12 Furthermore, the rapid and severe capillary leak that is characteristic of paediatric sepsis leads to hypovolaemia in the underresuscitated child, further reducing preload and cardiac output. For this reason, septic children often respond well to aggressive fluid resuscitation (> 60 ml/kg in the first hour). Peripheral oedema caused by leakage of proteinaceous fluid from injured capillary endothelia exacerbates cellular hypoxia by increasing the diffusion distance for oxygen between capillaries and cells.
MULTIPLE ORGAN FAILURE IN SHOCKED CHILDREN
DISTRIBUTIVE SHOCK
The main features are hypotension, vasodilatation and hypovolaemia due to plasma leakage from capillaries. The child’s extremities are warm and pink, the blood pressure is low and the pulse pressure is wide. There is tachycardia, oliguria and stupor. Other evidence of anaphylaxis (wheezing, urticaria, swollen face or tongue), spinal cord injury (bradycardia, quadriparesis, lax anal sphincter) or drug intoxication (history, tablets, coma out of proportion to shock) may be present (Table 101.6).
Table 101.6 Causes of distributive shock in childhood
MANAGEMENT
The child’s airway, breathing (see Chapter 98) and circulation should be secured during the initial assessment. The priorities in management of the circulation are to achieve:
ADEQUATE PERFUSION OF THE BRAIN AND HEART
This requires systolic and diastolic blood pressures of 80% of normal for age (see Table 101.3), achieved by aggressive early blood volume expansion and by pressor drugs. The conscious state is the best index of adequate brain perfusion, while improved coronary perfusion is shown by rising blood pressure with falling central venous pressure.
Preload
Pulmonary artery catheterisation for measurement of wedge pressure and cardiac output is rarely justified in children: evidence that it improves outcome is lacking,15 and side-effects occur commonly.16 Non-invasive monitoring of left and right ventricular preload by transthoracic echocardiography may be more useful than atrial pressure measurement, in that it removes the confounding factors of diastolic compliance and atrioventricular (AV) valve dysfunction, but it is subjective and operator dependent.17
Systemic vascular resistance
In septic or hypovolaemic shock, infusion of noradrenaline (0.05–2.0 μg/kg per min) should be started (via an intraosseous cannula when no central venous cannula is in place) if volume expansion does not achieve 80% of normal systolic blood pressure for age within 10 minutes. In septic shock, vasopressin (0.0003–0.0006 U/kg per min), the action of which does not depend on downregulated α1-receptors, may be used to reduce the dose of noradrenaline required. Vasopressin deficiency occurs commonly in septic shock18 and in irreversible shock of other causes.19

Full access? Get Clinical Tree