60 Severe Asthma Exacerbation
 Magnitude of the Problem
Magnitude of the Problem
Each year in the United States, acute asthma accounts for approximately 1.8 million emergency department visits, 497,000 hospitalizations, and 3800 deaths.1 All too commonly, failure to achieve adequate outpatient control lies at the crux of the problem. Asthma control is achieved in a minority of patients, largely due to the underuse of antiinflammatory agents, and poor control is a risk factor for asthma exacerbation.2 More than half of current asthmatics had one or more attacks during the preceding year, and there appears to be a subset of patients who are prone to exacerbations. Factors underlying the exacerbation-prone phenotype include cigarette smoking, medication nonadherence, psychosocial factors, poverty, obesity, and alterations in host cytokine response to viral infections.3 The rate of asthma death is higher in blacks than whites and in patients aged 65 and older. Patients who require mechanical ventilation for asthma have a mortality rate of less than 10% and are most likely to die of tension pneumothorax or nosocomial infection.4 Fortunately, the rate of asthma death (which had increased from 1980 to 1995) has decreased each year since 2000. Risk factors for fatal or near-fatal asthma are listed in Table 60-1.
TABLE60-1 Risk Factors for Fatal or Near-Fatal Asthma
 Pathophysiology of Acute Airflow Obstruction
Pathophysiology of Acute Airflow Obstruction
Regardless of the tempo of the attack, acutely ill asthmatics develop critical airflow obstruction. The time available for expiration (less than 2 seconds in a patient breathing 30/min) is insufficient for full exhalation, resulting in gas trapping and dynamic lung hyperinflation (DHI). Trapped gas elevates alveolar volume and pressure relative to mouth pressure at end-expiration, a state referred to as auto-PEEP.5 Auto-PEEP must be overcome by forcefully lowering pleural pressure during spontaneous inspiration, which increases the inspiratory work of breathing. At the same time, dynamic hyperinflation increases elastic work of breathing. Dynamic hyperinflation also decreases diaphragm force generation by placing the diaphragm in a mechanically disadvantageous position. Dynamic hyperinflation may be self-limiting because increases in lung volume increase lung elastic recoil pressure and airway diameter to augment expiratory flow. In the end, an imbalance between increased respiratory system load (both resistive and elastic) and decreased respiratory muscle strength may result in respiratory failure.6
 Clinical Features
Clinical Features
Dyspnea, cough, wheeze, and increased work of breathing are the hallmarks of acute asthma. Patients with moderate to moderately severe attacks are tachypneic and in mild to moderate respiratory distress. They have expiratory phase prolongation, difficulty speaking in long sentences, and audible wheezes. Arterial blood gases commonly reveal hypoxemia and acute respiratory alkalosis. A more severe attack leads to upright positioning, diaphoresis, monosyllabic speech, respiratory rate above 30/min, accessory muscle use, pulse above 120/min, pulsus paradoxus greater than 25 mm Hg, hypoxemia, and normo- or hypercapnia. Depressed mental status, paradoxical respiration, bradycardia, absence of pulsus paradoxus from respiratory muscle fatigue, and a quiet chest signal an impending arrest. The emergence of wheezes in these patients is generally a good marker that airflow has improved. Thus posture, speech, and mental status allow for a quick appraisal of severity, response to therapy, and need for intubation.7
 Emergency Department Disposition
Emergency Department Disposition
Asthmatic patients with inadequate response to albuterol in the ED invariably require hospital admission or prolonged treatment in an ED holding area (see later).8 Approximately one-third of patients are nonresponders to albuterol (Figure 60-1), which is not necessarily explained by prior heavy use of this medication. Rather, nonresponsiveness suggests a significant component of airway wall inflammation and the presence of intraluminal mucus. Albuterol nonresponders have negligible (i.e., <10%) changes in their PEFR after 30 to 60 minutes of therapy. These patients should be admitted to the hospital, as should patients with other markers of a severe attack such as a PEFR less than 40% of predicted or personal best PEFR, or deterioration despite ED treatment. Patients with respiratory failure, need for frequent albuterol treatments, fatigue, altered mental status, and cardiac arrhythmias require intensive care unit admission. Patients with an incomplete response to treatment in the ED, defined by improved but persistent symptoms and a PEFR or FEV1 between 40% and 69% of predicted, should be considered for admission, although selected patients safely return home with appropriate treatment and follow-up. Patients with a good response to treatment may be discharged home with appropriate instructions for anti-inflammatory therapy. These patients have a PEFR ≥ 70% an hour after their last treatment, a clear chest, and are in no distress.
 Pharmacologic Management
Pharmacologic Management
Selected drugs used in the treatment of acute asthma are presented in Table 60-2. Brief discussions of a few of the more common therapeutic agents employed to treat severe asthma exacerbation follow.
TABLE60-2 Selected Drugs Used in the Treatment of Acute Asthma
| Albuterol | 2.5 mg in 2.5 mL normal saline by nebulization every 15-20 min × 3 in the first hour or 4-8 puffs by MDI with spacer every 10-20 min for 1 hour, then as required; for intubated patients, titrate to physiologic effect and side effects. |
| Levalbuterol | 1.25 mg by nebulization every 15-20 min × 3 in the first hour, then as required. |
| Epinephrine | 0.3 mL of a 1 : 1000 solution subcutaneously every 20 min × 3. Terbutaline is favored in pregnancy when parenteral therapy is indicated. Use with caution in patients older than age 40 and in patients with coronary artery disease. |
| Corticosteroids | Methylprednisolone IV or prednisone PO 40-80 mg/d in 1 or 2 divided doses until PEFR reaches 70% of predicted or personal best. |
| Anticholinergics | Ipratropium bromide 0.5 mg (with albuterol) by nebulization every 20 min, or 8 puffs by MDI with spacer (with albuterol) every 20 min. |
| Magnesium sulfate | 2 g IV over 20 minutes, repeat once as required (total dose 4 g, unless hypomagnesemic). |
IV, intravenous; MDI, metered-dose inhaler; PEFR, peak expiratory flow rate; PO, per os (oral).
< div class='tao-gold-member'>

Full access? Get Clinical Tree


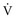 ) to perfused (
) to perfused (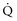 ) alveolar-capillary units. The severity of hypoxemia roughly tracks the severity of obstruction, but in recovering patients, airflow rates may improve faster than Pa
) alveolar-capillary units. The severity of hypoxemia roughly tracks the severity of obstruction, but in recovering patients, airflow rates may improve faster than Pa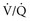 inequality, indicating that larger airways recover faster than smaller airways. Multiple inert gas elimination technique (MIGET) analysis also demonstrates small areas of high
inequality, indicating that larger airways recover faster than smaller airways. Multiple inert gas elimination technique (MIGET) analysis also demonstrates small areas of high 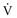 relative to
relative to 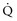 and slightly increased physiologic dead space in acute asthma. This may result from decreased blood flow to hyperinflated lung units. Elevated dead space and decreased minute ventilation in the critically hyperinflated and fatiguing patient underlie the development of hypercapnia in severe exacerbations.
and slightly increased physiologic dead space in acute asthma. This may result from decreased blood flow to hyperinflated lung units. Elevated dead space and decreased minute ventilation in the critically hyperinflated and fatiguing patient underlie the development of hypercapnia in severe exacerbations.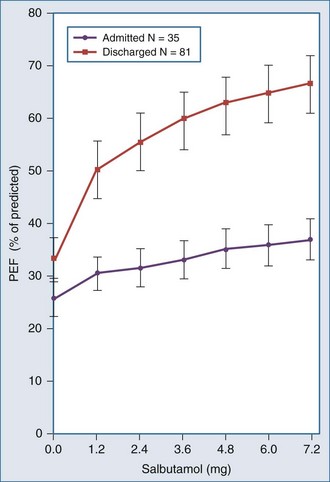
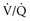 units. Oxygen saturation should be monitored until there is clear clinical progress, remembering that improved oxygenation may lag behind improved airflow rates.
units. Oxygen saturation should be monitored until there is clear clinical progress, remembering that improved oxygenation may lag behind improved airflow rates.

