Fig. 1
Provoked central sensitization. Central sensitization can be provoked by intradermal injection of capsaicin. The boundary and hence the area of secondary pinprick hyperalgesia can be assessed by von Frey hair stimulation (change in intensity of the provoked pin prick pain). The boundary and hence the area of dynamic mechanical allodynia can be determined as the change in the perception provoked by brushing the skin from a non-painful sensation to a painful sensation. The areas of hyperalgesia and/or allodynia can be enlarged in chronic pain patients with central sensitization as an indication of increased central gain
The intradermal capsaicin models have also been used to show increased hyperalgesic reactions in patients with rheumatoid arthritis (Morris et al. 1997), fibromyalgia (Morris et al. 1998), and vulvodynia (Foster et al. 2005).
The capsaicin model has been applied to non-painful conditions such as patients with multiple chemical sensitivity and showed facilitated hyperalgesic reactions (Holst et al. 2011).
Transdermal electrical stimulation is another way to induce central sensitization, and larger hyperalgesic areas have been shown in patients with chronic lumbosacral pain including radicular neuropathic features as compared to controls (Lecybyl et al. 2010).
One study has used the capsaicin model in patients for pharmacological profiling. Patients with unilateral sciatica showed heightened responses to intradermal capsaicin compared to pain-free volunteers, and the study by Sumracki et al. (2012) compared the effects of pregabalin (300 mg) and the tetracycline antibiotic and glial attenuator minocycline (400 mg) on capsaicin-induced spontaneous pain, flare, and allodynia and between the affected and unaffected leg. It was concluded that a healthy control group was needed for such studies in order to show differences as a pain patient cannot be used as his/her own control.
Temporal summation and aftersensation (Fig. 2 ): An important and potent central mechanism in dorsal horn neurons is the temporal integration also termed wind-up-like pain. The initial phase of the wind-up process observed in animals translates into temporal summation in humans (Arendt-Nielsen et al. 1994). If a painful stimulus is repeated 1–3 times per second, the pain will integrate and become more painful with an intensity and stimulus frequency-dependent summation (Arendt-Nielsen et al. 1994). Temporal summation can be elicited using electrical, mechanical, or thermal stimulation modalities and is elicited from the skin, musculoskeletal structures, and viscera (Arendt-Nielsen 1997; Arendt-Nielsen and Yarnitsky 2009).
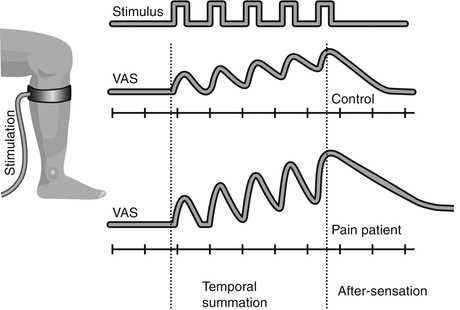

Fig. 2
Temporal summation and aftersensation. If a painful stimulus (in this case a cuff inflated around the leg) of the same intensity is repeated, e.g., five times with 2 s intervals, the pain intensity will gradually increase as assessed on a visual analogue scale (VAS). This is called temporal summation and is a central phenomenon. In a chronic pain patient with central sensitization, the temporal summation is facilitated, and in some cases, the pain will continue for some seconds after the stimulation has been terminated (aftersensation)
In many clinical conditions when repeated stimuli are applied in neuropathic, musculoskeletal, and visceral chronic pain patients, temporal summation is substantially facilitated (Arendt-Nielsen et al. 1997a; Graven-Nielsen et al. 2000; Nikolajsen et al. 1996).
In clinical bed site testing, simple devices are used for assessing temporal summation such as tapping the skin with a nylon filament (Nikolajsen et al. 1996). When more standardization is required, however, automated user-independent methods are needed such as thermal (Kong et al. 2013), mechanical (Nie et al. 2009), or electrical stimulation techniques of the skin (Arendt-Nielsen et al. 1994), muscles (Arendt-Nielsen et al. 1997b), or viscera (Drewes et al. 1999). The abovementioned cuff-algometry technique can also be used if the cuff is repeatedly inflated (Lemming et al. 2012; Skou et al. 2013).
When repeated stimuli are delivered, sometimes pain patients experience an aftersensation (pain after the stimulus has stopped) (Robinson et al. 2010). This has been observed in patient with neuropathic (Gottrup et al. 2003) and musculoskeletal pain (Staud et al. 2003, 2007). An exclusive facilitation of the aftersensation alone has been proposed to be of diagnostic value (Sato et al. 2012) and supports the basic finding that the summation and the aftersensation are mediated by different underlying central mechanisms (Price et al. 1978; You et al. 2005).
Facilitated temporal summation in chronic pain patients is efficiently inhibited by NMDA receptor antagonists (ketamine and amantadine). This has been found in patients with surgical incisions (Stubhaug et al. 1997), postherpetic neuralgia (Eide et al. 1994), phantom limb pain (Nikolajsen et al. 1996), chronic postsurgical neuropathic pain (Pud et al. 1998), and fibromyalgia (Graven-Nielsen et al. 2000).
Abnormal temporal summation in patients with neuropathic pain does not predict the clinical effect of imipramine or gabapentin (Rasmussen et al. 2004).
Some clinical studies have not shown effect on facilitated temporal summation by lamotrigine (an antiepileptic drug acting on voltage-sensitive sodium channels) in patients with spinal cord injury pain (Finnerup et al. 2002) and by the NMDA-antagonist memantine in patients with phantom limb pain (Nikolajsen et al. 2000) and postherpetic neuralgia (Eisenberg et al. 1998).
More clinical studies are needed to understand which compounds can modulate temporal summation.
Spatial summation (Fig. 3 ): Spatial summation is another mechanism, which relies on central networks and the general sensitization status (Bouhassira et al. 1995). In humans, spatial summation can be assessed in different ways where the stimulus is applied to using different stimulation areas, e.g., using thermodes (Nielsen and Arendt-Nielsen 1997), pressure probes (Nie et al. 2009), or cuff algometry (Polianskis et al. 2002). The cuff-algometry technology can utilize one or two cuffs which are automatically inflated, and the volunteer/patient rates the provoked pain intensity (Polianskis et al. 2002). Spatial summation is facilitated in various pain conditions such as fibromyalgia (Staud et al. 2004, 2007), osteoarthritis (Graven-Nielsen et al. 2012), and lateral epicondylitis (Jespersen et al. 2013).
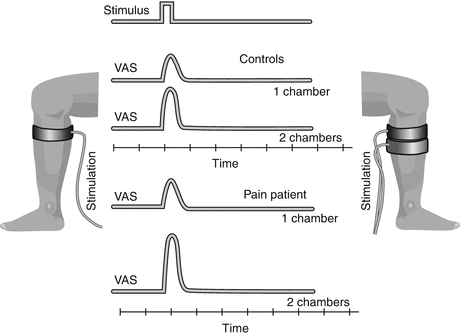

Fig. 3
Spatial summation. The pain intensity provoked by a single stimulus (in this case a cuff inflated around the leg) can be assessed on a VAS. If the area of the stimulation is increased (stimulating with two cuffs instead of one cuff), the pain intensity will increase. This is called spatial summation and is a central phenomenon. In a chronic pain patient with central sensitization, the spatial summation may be facilitated (increased ratio between the pain intensity provoked by the large area and the pain intensity provoked by the small area)
No pharmacological screening studies have used this technique in pain patients.
Reflex receptive fields (Fig. 4 ): In many animal studies, expansion of receptive fields of dorsal horn neurons has been documented in neuropathic as well as inflammatory (cutaneous, muscle, and viscera) models. Expansion of receptive fields has been a challenging mechanism to assess in humans. In animals, an alternative method for assessment of receptive field expansion of dorsal horn neurons has been developed. This involves quantification of the so-called reflex receptive field (Schouenborg et al. 1994, 1995) which was found to expand in the presence of central sensitization (Harris and Clarke 2003).
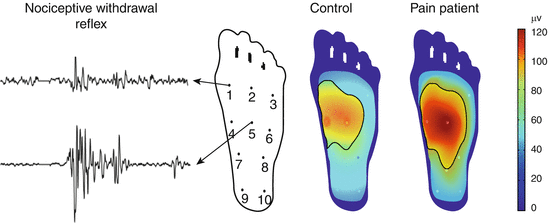

Fig. 4
Reflex receptive fields. Nociceptive withdrawal reflexes evoked by electrical stimulation from ten individual points on the sole of the foot. The reflex recorded from, e.g., the tibialis anterior muscle is large when evoked from, e.g., point 5 and smaller when elicited from, e.g., point 1. Based on the size of the reflex, the so-called reflex receptive field can be constructed and can be found larger in chronic pain patients with central sensitization
This method based on assessment of the nociceptive withdrawal method has been translated to humans (Andersen et al. 2001), and the reflex receptive field is found to be expanded into spinal cord injured patients (Andersen et al. 2004), in patients with chronic visceral pain (endometriosis) (Neziri et al. 2010), in patients with low back pain (Biurrun Manresa et al. 2013), and in patients with neck pain (Biurrun Manresa et al. 2013).
To date, few pharmacological studies have used this technique for profiling, and the sensitivity is not known. One study showed that the 5-HT-3 receptor antagonist had no effect on the expanded fields in chronic low patients and no effect on the clinical pain (Neziri et al. 2012). This advanced technique may in the future provide new information in clinical studies and for profiling of new compounds.
Descending pain modulation (Fig. 5 ): There are increasing evidences that the balance between the descending pain inhibition and facilitation may be disturbed in chronic pain and that this phenomenon has a role in maintaining central sensitization (Porreca et al. 2002; Suzuki et al. 2004; You et al. 2010). In humans, status assessment of the descending pathways has recently undergone a revival, and the original DNIC terminology has been renamed to conditioning pain modulation (CPM, Yarnitsky et al. 2010). Less efficient CPM seems to be a general phenomenon in chronic pain conditions (Staud 2012) such as patients with, e.g., myofascial temporomandibular joint pain (Bragdon et al. 2002), chronic low back pain (Peters et al. 1992), fibromyalgia (Kosek and Hansson 1997), whiplash (Daenen et al. 2014), painful OA (Arendt-Nielsen et al. 2010), and chronic tension-type headaches (Sandrini et al. 2006), chronic pancreatitis, and interstitial cystitis (Ness et al. 2014). OA patients with a deficient, descending pain inhibition show a normalization to a pain-free state after surgery (Graven-Nielsen et al. 2012; Kosek and Ordeberg 2000) suggesting that the chronic pain maintained the CPM dysfunction and that the chronic pain saturated the CPM mechanism so the conditioning pain stimulus is less efficient.
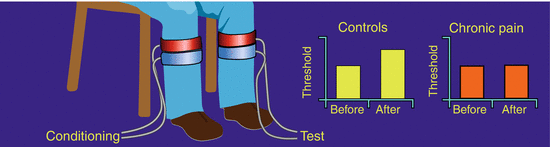

Fig. 5
Descending pain modulation. The descending pain modulation can be assessed by delivering a tonic conditioning pain stimulation to one location (in this case cuff stimulation of the leg), e.g., 2 min. A test stimulus is applied to another location, and the response (e.g., a pain threshold) is assessed before and during the conditioning stimulation. In healthy controls, the threshold increases during the conditioning stimulation (pain inhibition), but in chronic pain patients with central sensitization, this increase is smaller or even absent
The CPM paradigm has in recent years been implemented in pharmacological screening studies, and modulatory effects can be induced by dexmedetomidine (a selective α(2)-adrenoceptor agonist) (Baba et al. 2012), buprenorphine (Arendt-Nielsen et al. 2012a, b), fentanyl (Arendt-Nielsen et al. 2012a, b), duloxetine (Yarnitsky et al. 2012), and apomorphine (a non-specific dopamine agonist) (Treister et al. 2013), whereas oxycodone seems not to modulate CPM (Suzan et al. 2013).
The use of CPM for assessing drug efficacy in pain patients has only been used in one study so far where the effect of duloxetine in painful diabetic neuropathy was investigated (Yarnitsky et al. 2012).
Evidently, an alteration in the descending pain modulation could be a promising target for pharmacological intervention (Arendt-Nielsen and Yarnitsky 2009) and should be utilized when new pain drugs are evaluated.
Offset analgesia (Fig . 6 ): Offset analgesia is provoked by incremental decreases of a nociceptive heat stimulus, and the perception of the provoked pain is less as what is provoked when the same stimulus intensity is given as a single stand-alone stimulus (Grill and Coghill 2002; Yelle et al. 2008). A peripheral component of offset analgesia cannot be excluded but is generally considered as a central inhibitory modulation of pain and as such should be modulated by central sensitization. This is supported by the fact that an association between CPM and offset analgesia has been shown indicating some commonalities of their underlying mechanisms (Honigman et al. 2013).
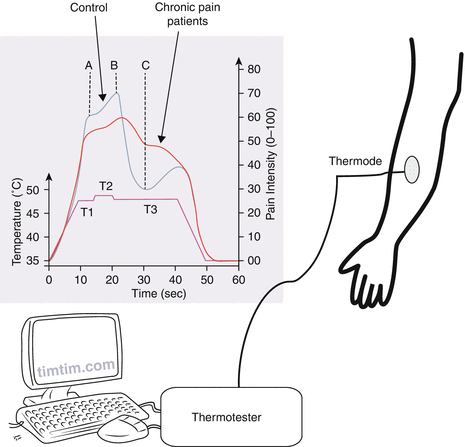

Fig. 6
Offset analgesia. The thermal stimulus paradigm used to evoke offset analgesia. The first phase (T1) consists of a painful heat stimulus applied for 5 s. The second phase (T2) is 1° above T1 also for 5 s. The last phase (T3) is the same temperature as T1 but for 20 s. During the stimulation, the person rates the pain intensity (0–100). The pain rating during T3 is lower than to T1 although the stimulus intensity is the same. This reduction in pain intensity (between B and C) is called offset analgesia. Chronic pain patients have a less potent offset analgesic response as compared with controls
In healthy volunteers, offset analgesia is found stable in one study (Nilsson et al. 2013) but in another study found to be facilitated when assessed in repeated sessions. Furthermore, the response is reduced in potency with age (Naugle et al. 2013). Induction of thermal hyperalgesia has shown an association with increased magnitude of offset analgesia (Martucci et al. 2012a, b), whereas other studies have tried to modulate the potency of offset analgesia by experimental central sensitization without success (Martucci et al. 2012a, b), suggesting that longer-lasting central sensitization is needed.
Only one clinical study has used the offset analgesia paradigm in patients with neuropathic pain and found the response was reduced or absent (Niesters et al. 2011a, b). More clinical studies are needed to confirm the usefulness of this technique.
The offset analgesia is not modulated by intravenous ketamine (Niesters et al. 2011a, b) or morphine (Martucci et al. 2012a, b). More studies are needed to understand the role of this technique in pharmacological screening.
Referred pain (Fig. 7 ): Referred pain is a central phenomenon predominantly related to pain from deep somatic and visceral structures. Referred pain areas are facilitated as a result of a central sensitization in patients with, e.g., fibromyalgia (Sorensen et al. 1998), whiplash (Koelbaek Johansen et al. 1999), osteoarthritis (Arendt-Nielsen and Yarnitsky 2009), chronic low back pain (O’Neill et al. 2007), irritable bowel syndrome (Swarbrick et al. 1980), and chronic pancreatitis (Dimcevski et al. 2007). In addition, it has been shown that referred pain areas evoked from muscles are enlarged in visceral pain conditions (Bajaj et al. 2003) as a result of convergence and interaction between sensitization related to deep somatic and visceral structures but also manifested as viscero-visceral sensitization (Giamberardino et al. 2010). Patients with, e.g., urinary calculosis show significant and long-lasting hyperalgesia in the referred pain areas (Giamberardino et al. 1994); patients suffering from a large number of colics show more hyperalgesia than those experiencing a limited number of colics. Similar somatic hyperalgesia is found in referred pain areas in acute appendicitis (Stawowy et al. 2002) and cholecystolithiasis (Stawowy et al. 2005).
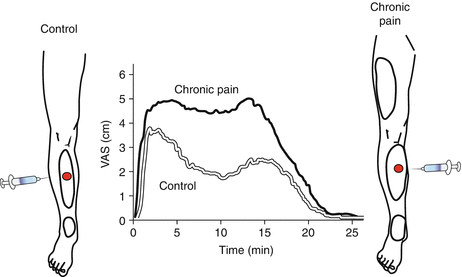

Fig. 7
Referred pain. Local and referred pain can be provoked by intramuscular injection of a bolus of an algogenic substance (e.g., 6 % hypertonic saline). Local pain area develops around the injection site and referred pain develops around a distal location in healthy controls. Referred pain is a central phenomenon. In chronic pain patients with central sensitization, the referred pain area is larger and can spread also to proximal locations. The intensity of the pain provoked is rated stronger by chronic pain patients with central sensitization as compared with controls
Few studies have used the referred pain areas in pain patients for pharmacological profiling. Ketamine (Arendt-Nielsen and Graven-Nielsen 2008), gabapentin (Arendt-Nielsen et al. 2007), and a 5-HT antagonist (Christidis et al. 2008) are found to reduce the area of referred pain. On the contrary, alfentanil and morphine do not reduce referred pain (Schulte et al. 2006).
4 Conclusion and Future Perspectives
Central sensitization can manifest in many different ways, and it is evident that a localized nociceptive focus over time can affect the entire neuroaxis. Development of new, safe, centrally acting compounds targeting central sensitization has not been successful, and new strategies have been taking towards the peripheral target and towards the nonneuronal components.
A number of mechanism-based QST approaches have been developed to assess the potency of the central sensitization mechanisms. Sensory testing can be used to profile chronic pain patients and as a screening tool in a more efficient development of new analgesic compounds.
Profiling chronic pain patients by mechanistic QST in conjunction with pharmacological treatment opportunities may in the future provide the basis for individualized, targeted pain management.
Conflict of Interest
No conflict of interest can be reported.
References
Andersen OK, Sonnenborg FA, Arendt-Nielsen L (2001) Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clin Neurophysiol 112(4):641–649PubMed
Andersen OK, Finnerup NB, Spaich EG, Jensen TS, Arendt-Nielsen L (2004) Expansion of nociceptive withdrawal reflex receptive fields in spinal cord injured humans. Clin Neurophysiol 115(12):2798–2810PubMed
Arendt-Nielsen L (1997) Induction and assessment of experimental pain from human skin, muscle and viscera. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z (eds) Proceedings of the 8th World Congress on pain, Vancouver, Canada, August 17–22: progress in pain research and management, vol 8. IASP Press, Seattle, pp 393–425
Arendt-Nielsen L, Curatolo M (2013) Mechanistic, translational, quantitative pain assessment tools in profiling of pain patients and for development of new analgesic compounds. Scand J Pain 4(4):226–230
Arendt-Nielsen L, Hoeck HC (2011) Optimizing the early phase development of new analgesics by human pain biomarkers. Expert Rev Neurother 11(11):1631–1651PubMed
Arendt-Nielsen L, Yarnitsky D (2009) Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 10(6):556–572PubMed
Arendt-Nielsen L, Brennum J, Sindrup SH, Bak P (1994) Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur J Appl Physiol Occup Physiol 68:266–273PubMed
Arendt-Nielsen L, Drewes AM, Hansen JB, Tage-Jensen U (1997a) Gut pain reactions in man: an experimental investigation using short and long duration transmucosal electrical stimulation. Pain 69(3):255–262PubMed
Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Jensen TS (1997b) Temporal summation in muscles and referred pain areas: an experimental human study. Muscle Nerve 20(10):1311–1313PubMed
Arendt-Nielsen L, Frokjaer JB, Staahl C, Graven-Nielsen T, Huggins JP, Smart TS, Drewes AM (2007) Effects of gabapentin on experimental somatic pain and temporal summation. Reg Anesth Pain Med 2(5):382–388
Arendt-Nielsen L, Graven-Nielsen T (2008) Muscle pain: sensory implications and interaction with motor control. Clin J Pain 24(4):291–298PubMed
Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T (2010) Sensitization in patients with painful knee osteoarthritis. Pain 149(3):573–581PubMed
Arendt-Nielsen L, Andresen T, Malver LP, Oksche A, Mansikka H, Drewes AM (2012a) A double-blind, placebo-controlled study on the effect of buprenorphine and fentanyl on descending pain modulation: a human experimental study. Clin J Pain 28(7):623–627PubMed
Arendt-Nielsen L, Graven-Nielsen T, Petrini L (2012b) Experimental human models and assessment of pain in non-pain conditions. In: Giamberardino MA, Jensen TS (eds) Pain comorbidities: understanding and treating the complex patient. IASP Press, Seattle, Chapter 3
Atzeni F, Cazzola M, Benucci M, Di Franco M, Salaffi F, Sarzi-Puttini P (2011) Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol 25(2):165–171PubMed
Aykanat V, Gentgall M, Briggs N, Williams D, Yap S, Rolan P (2012) Intradermal capsaicin as a neuropathic pain model in patients with unilateral sciatica. Br J Clin Pharmacol 73(1):37–45PubMedCentralPubMed
Baba Y, Kohase H, Oono Y, Fujii-Abe K, Arendt-Nielsen L (2012) Effects of dexmedetomidine on conditioned pain modulation in humans. Eur J Pain 16(8):1137–1147PubMed
Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L (2001a) Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 93(2):107–114PubMed
Bajaj P, Graven-Nielsen T, Arendt-Nielsen L (2001b) Trigger points in patients with lower limb osteoarthritis. J Musculoskelet Pain 9:17–33
Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L (2003) Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain 4(7):372–380PubMed
Balasubramaniam R, de Leeuw R, Zhu H, Nickerson RB, Okeson JP, Carlson CR (2007) Prevalence of temporomandibular disorders in fibromyalgia and failed back syndrome patients: a blinded prospective comparison study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:204–216PubMed
Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M (2004) Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 107:7–15PubMed
Baron R, Saguer M (1994) Axon-reflex reactions in affected and homologous contralateral skin after unilateral peripheral injury of thoracic segmental nerves in humans. Neurosci Lett 165(1–2):97–100PubMed
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284PubMedCentralPubMed

Full access? Get Clinical Tree






