More conservative treatment options have failed, or other options are unacceptable or not indicated.
A trial of neuraxial medications provides acceptable pain relief, tolerable side effects, and functional improvement, when indicated.
Oral or transdermal medications produce unacceptable side effects or an unacceptable level of relief.
The patient’s spinal anatomy will allow for the placement of a spinal catheter.
The patient is medically stable, with no untreated bleeding disorders.
The patient is medically stable, with no untreated infectious processes.
The patient has no skin disorders that would preclude the implantation of a foreign body.
The patient is mentally stable, with no untreated severe depression or anxiety disorders.
No significant personality disorder, such as borderline or antisocial personality disorder, has been diagnosed in the patient.
The region of pain can be covered by the anatomic congruent placement of an intrathecal catheter.
Importantly, the intrathecal pump functions as a platform to deliver medication into the intrathecal space in an effort to treat pain or spasticity. (For the purposes of this chapter, we are focusing on patient selection for pain management.) Therefore, it does not define the medication selected, but simply provides a method of delivering it more sustainably. This paradigm—separating pump from medicine—is advantageous to note. Further, patient selection is also centered on a careful, algorithmic approach to intrathecal therapy, taking into account the type and location of the pain (Fig. 36.1). Although historically intrathecal therapy has been positioned as salvage therapy after trials of stimulation therapies, recently intrathecal therapy has been entering into the algorithm concurrent with other neuromodulation strategies.
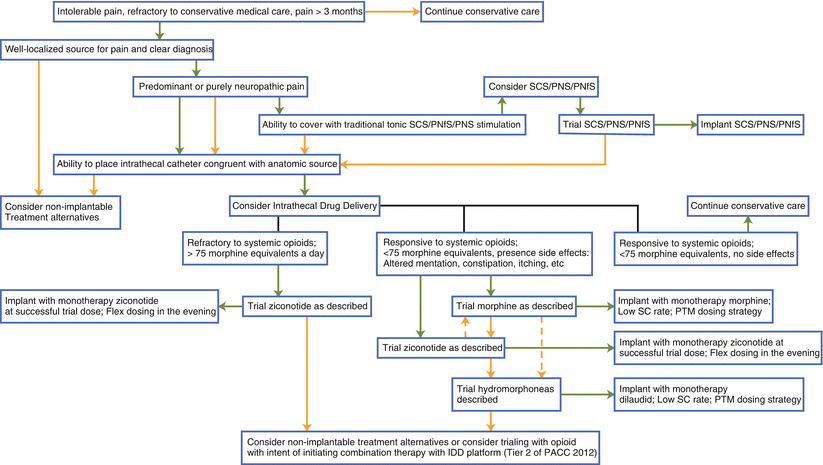

Fig. 36.1
Algorithmic approach to advanced pain care therapies. Green arrows indicate affirmative position; orange arrows indicate deaffirmative position; hashed arrows indicate physician preference
36.2 Factors to Determine Proper Indications for Pump Implantation
Intrathecal pumps are indicated for chronic use in patients with moderate to severe pain of cancer and noncancer origin. The indications for these devices vary based on the disease process and the effect of the disease on the source of pain generation. Some of the more common indications are listed in Table 36.2.








