1. Anesthesiologists remain in key position to protect and further advance patient safety.
2. Patient safety organizations (PSOs) provide federally protected venue to accumulate patient safety information and make recommendations on a national level.
3. While the overall mortality for all age group has decreased over the past decades, infants remain at higher risk for suffering critical incidents.
4. The risk of anesthetics needs to be weighed carefully against comorbidities and acute changes in usual health.
PATIENT SAFETY AND QUALITY IMPROVEMENT ARE INTEGRAL TO ANESTHESIOLOGY. In fact, it was an anesthesiologist, E. C. Pierce Jr, who first used the term patient safety in 1984.
Ingrained vigilance and preoccupation with failure are hallmarks of high reliability organizations (HROs), a goal that health care is only striving to reach. The reality is that adverse events and medical errors happen every day. Rather than denying and covering up these events, there is much we can learn from them, and, like HROs, the specialty of anesthesiology is embracing reporting, event investigation, and contributions to the “just culture” as key tools to improving care.
Incident analysis looks both back and forward; it enables one to understand what led to a specific event and affords an opportunity to recognize system weaknesses that could recreate the same or similar situation for future patients (1). A reliable reporting culture is essential to assess the true risk of our anesthetics. However, the current voluntary reporting systems likely underestimate the true morbidity and mortality rates of anesthetics, and one only hopes that with the ever improving technology, this type of data capture will become more reliable (2). Furthermore, our assessments of anesthetic complications look only at events immediately proximate to the anesthetic and not at long-term outcomes. The implementation of systems to collect data from electronic medical records, which can then be screened, evaluated, and categorized by expert reviewers, will be essential in determining more reliable estimates of the true incidence of adverse events in our patients. While these observational data can never be used to prove causality, well-chosen outcome indices can help drive process and care improvement. The availability of these data will also be the key to long-term follow-up. Unfortunately, the data from most of our current technology lack the reliability and sophistication necessary for truly meaningful interpretation, but these systems are rapidly advancing and improving.
In the US, patient safety organizations such as the Anesthesia Quality Institute (AQI) or Wake Up Safe (WUS) enjoy federal protection and play significant role for the future in collecting large amount of information from many institutions to detect trends, evaluate the circumstances of rare events, and make recommendations for safer practice. Emerging collaborative efforts are seeking to acquire large scale data to estimate risk, develop benchmarks, and examine the circumstances surrounding rare adverse or near miss events, such as the Multicenter Perioperative Outcomes Group (MPOG), which aggregates the raw data from vast numbers of anesthetics, the Anesthesia Incident Reporting System (AIMS) of AQI, which provides online software to anonymously report de-identified events to a national repository, and various specialty-specific registries, such as the Pediatric Difficult Intubation Registry (PeDIR) and the Pediatric Regional Anesthesia Network (PRAN). We can expect that leveraging this kind of information may provide insight to better delineate both best and hazardous practices.
CLINICAL PEARL Departmental quality improvement programs can be enhanced and supplemented by participating in national registries and reporting systems, which can provide structure and benchmark data.
Risk is defined as the chance or possibility of danger, loss, or injury (3). Since the earliest surveys of anesthetic mortality and morbidity, the first decade of life was clearly identified as the greatest risk period, with an incidence of cardiac arrest as high as 13.9:10,000 (4). One fifth of the deaths occurred within the first week of life.
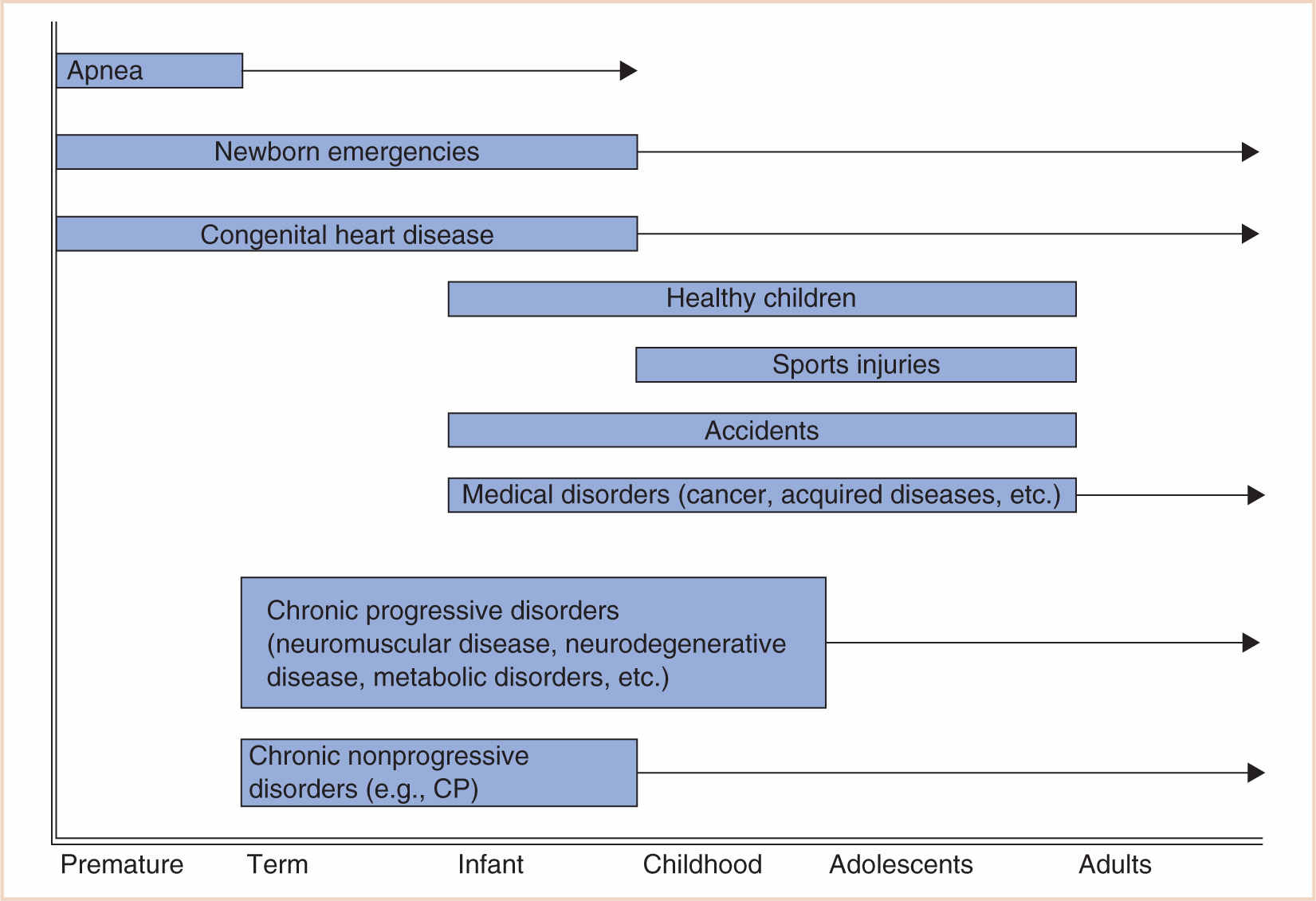
In pediatric anesthesia, this increased risk may be due to several reasons: the age of the child, existing comorbidities, physiologic stability and developmental fragility, and the urgency of the procedure. While most of these factors are out of the control of the anesthesiologist, recognizing the risk will enable one to prepare, manage, and ameliorate or prevent the consequences of adverse outcomes (5) (Fig. 4.1).
In 2001, Morita et al. reported 297 critical incidents in 10,000 prospectively evaluated anesthetics. Most of them occurred in healthy subjects (80.1% American Society Anesthesiologists [ASA] I and II) scheduled for elective surgery (73.3%). Critical incidents in infants younger than 1 year were four times as common as in older children (8.6% vs. 2.1%) and occurred mainly during the maintenance phase of anesthesia (80.6%). There was no anesthetic mortality. Respiratory events were the most common (77.4%) with laryngospasm accounting for 35.7%. Cardiovascular incidents (10.8%) included hypotension from hemorrhage, sepsis, and dysrhythmias (6).
In 2004, Murat et al. (7) reported that even in a pediatric hospital, age-stratified adverse events continue to occur in a pattern similar to other reports. Respiratory events constitute most of the adverse events, more frequently in infants and in relation to otorhinolaryngology (ORL) surgery. Cardiac events comprised 12.5% of intraoperative adverse events and occurred mainly in children with an ASA Physical Status (PS) score of 3 to 5. Vomiting was the most frequent adverse event in the postanesthetic care unit (PACU) and occurred more often in older children.
Examining four large audits in 2010, Paterson came to similar conclusions. He found that anesthesia-related cardiac arrest most commonly occurs in infants, in patients of ASA PS 3 to 5 and in those undergoing emergency procedures (3). This newer study also showed that overall morbidity (and in all likelihood, mortality) has been significantly reduced by the decreasing use of halothane and highlighted the benefits of continuous pulse oximetry and capnography as standard monitors.
CLINICAL PEARL Critical incidents are severalfold higher in infants under a year of age.
CARDIAC RISKS AND ADVERSE EVENTS
MORTALITY
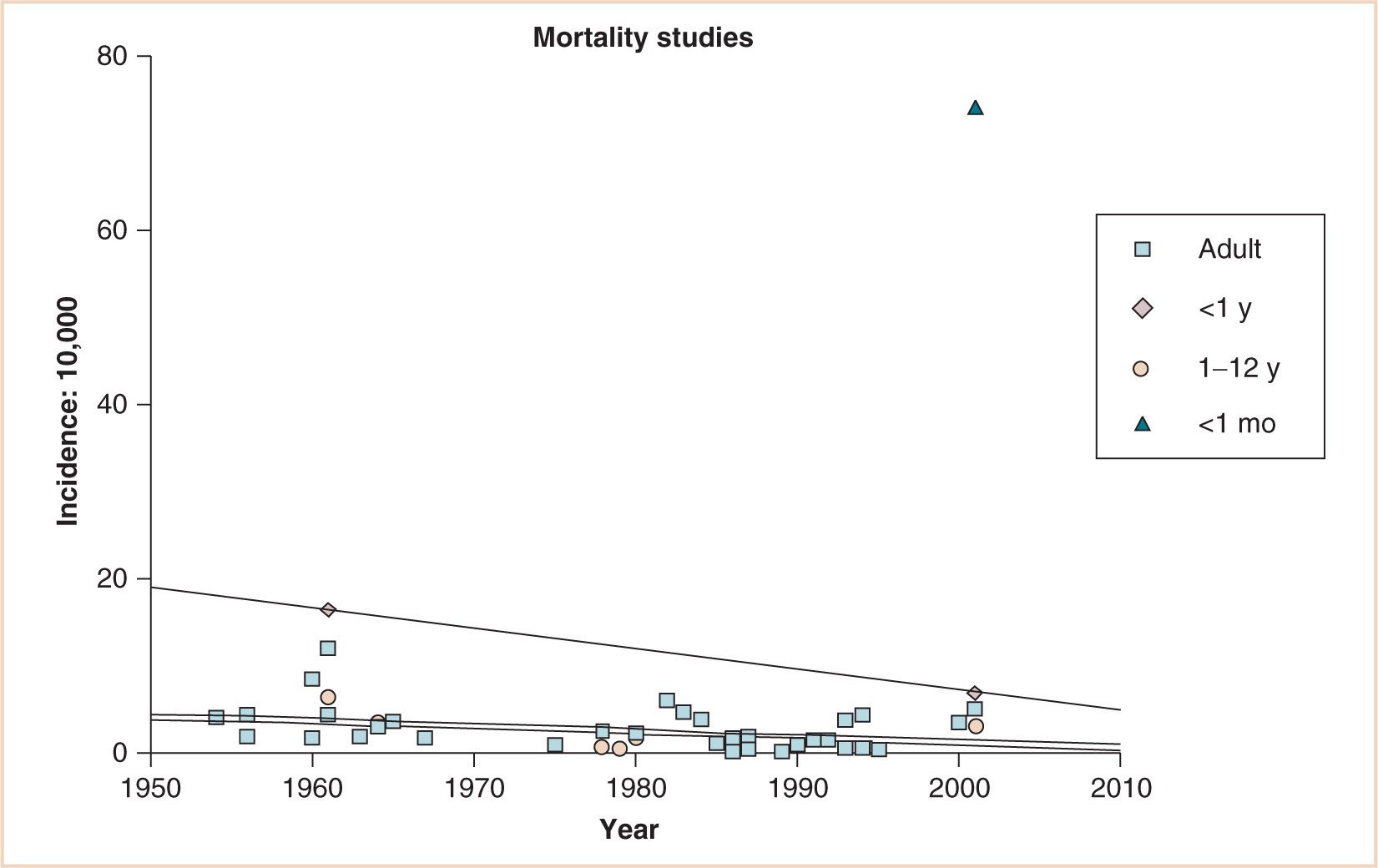
Although the overall adult and child (1 to 12 years of age) mortality rate is substantially less over 60 years of various retrospective, prospective, and audit studies, a substantial decrease has occurred for infants younger than 1 year of age. There remains a huge gap, however, for neonates younger than 1 month of age (1, 3–42) (see Fig. 4.2).
CARDIAC ARREST
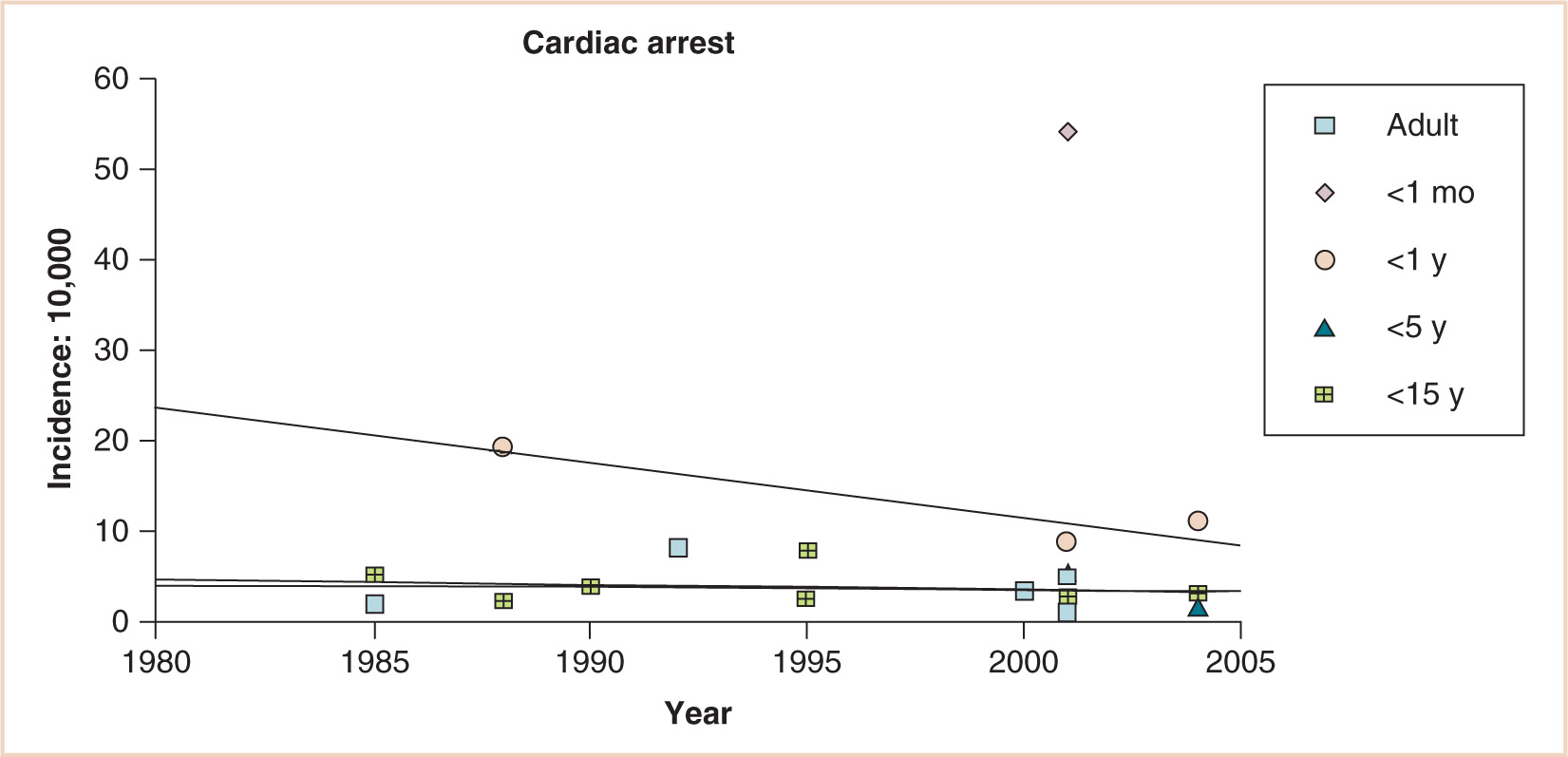
The virtually parallel data points for children younger than 15 and even younger than 5 years, the decreasing incidence of cardiac arrest for infants younger than 1 year, and the persistent gap between these infants and neonates younger than 1 month suggest substantial differences in anesthetic risk for young infants (see Fig. 4.3). Although lacking a denominator, 289 cases of cardiac arrest were reported during the first 4 years of the pediatric perioperative cardiac arrest (POCA) registry; 150 were related to anesthesia. Cardiac arrest related to anesthesia had an incidence of 1.4:10,000 and a mortality rate of 26%. Medication-related (37%) and cardiovascular (32%) causes of cardiac arrest were most common, together accounting for 69% of all arrests. Cardiovascular depression from halothane, alone or in combination with other drugs, was responsible for two thirds of all medication-related arrests. Thirty-three percent of the patients were ASA PS 1 to 2; in this group, 64% of arrests were medication related, compared with 23% in PS 3 to 5 patients. Infants younger than 1 year accounted for 55% of all anesthesia-related arrests. Multivariate analysis demonstrated two predictors of mortality: PS 3 to 5 (odds ratio, 12.99; 95% confidence interval, 2.9 to 57.7) and emergency status (odds ratio, 3.88; 95% confidence interval, 1.6 to 9.6) (8). Newer data from the 2007 POCA registry report a decline in halothane-induced cardiac arrest concomitant with the declining use of that drug in the United States (9). Still, meticulous attention to the depth of anesthesia during induction is critical to avoid iatrogenic cardiac depression, especially in the fasted child with relatively diminished circulating volume.
CLINICAL PEARL Drug-related causes of cardiac arrest are more common in healthy compared with sick infants, although their incidence has declined coincident with the disuse of halothane in the United States.
Functional (innocent) murmurs are nonpathological and are present in up to 20% of children at some time during childhood. They must be distinguished from murmurs caused by structural heart disease. The presence of congenital heart disease is of great importance to the anesthesiologists while the incidence of functional murmurs is high; thus, the pediatric anesthesiologists must be skilled in their diagnosis, yet when the nature of a murmur is in doubt, a well-organized system should be in place so that it can be thoroughly and efficiently investigated (10).
Anesthetic management of the child with congenital heart disease for noncardiac surgery relies on understanding the underlying residual pathophysiology (11–13). Additional special considerations for children who have uncorrected, palliated, or corrected congenital heart disease include a propensity to cerebrovascular accidents (14), altered ventilatory control and ventilatory mechanics (15,16), neurobehavioral consequences of the underlying disease (17), chronic elevation of endogenous catecholamine levels with congestive heart failure that return to normal after successful therapy (18,19), and possible electrophysiological abnormalities (e.g., prolonged QT, atrial and ventricular conduction defects) as a result of progression of the disease or previous surgical intervention (20). It is particularly important to remember that children will not always respond in an identical manner as adults given the same presenting signs, and it is risky to treat them the same way without specific knowledge about pediatric cardiac disease. The postcardiac transplant patient requires a sound understanding of the management of the denervated heart (21). Children demonstrate remarkable adaptability following surgical correction, however, and may recover significantly from an earlier, poorly functional cardiac status (22,23) (see Fig. 4.4).
Strafford (24) reported uneventful outcomes in 25 children undergoing day surgery, the majority following palliative or corrective cardiac surgery; but despite an increasing population of second- and third-decade survivors of congenital heart surgery, the noncardiac surgical outcomes of this “aging” population lack any substantial large denominator study. Therefore, for the time being, individualization of care by experienced anesthesiologists in tertiary or quaternary care centers with substantial support from experts will be best (2).
RESPIRATORY ADVERSE EVENTS
Pediatric patients have diminished oxygen reserves, due to lower functional residual capacity and lower closing volumes. This makes children and especially neonates susceptible to develop hypoxemia faster and to a lager degree than adults. They have more irritable airways and more frequent viral respiratory illnesses, and many of the most common operations in children (e.g., adenotonsillectomy, myringotomy and tube placement, microlaryngoscopy and bronchoscopy) involve the airway. Therefore, it is not surprising that respiratory events are more common and the morbidity and mortality rates are greater in pediatric claims, resulting in death (70%) or significant brain injury (30%) compared with adults (10).
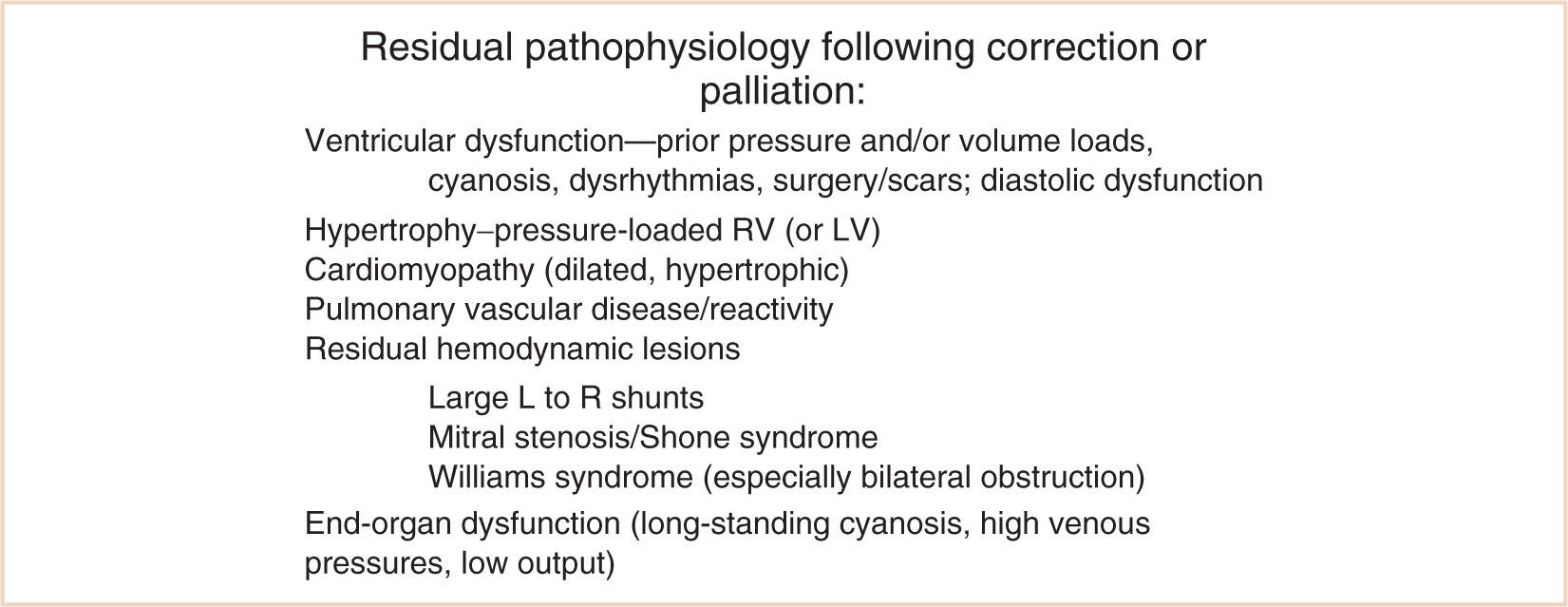
FIGURE 4.4 Residual pathophysiology following correction or palliation. RV, right ventricle; LV, left ventricle; L, left; R, right.
Laryngospasm, bronchospasm, and aspiration occur more often in children than in adults, and in even greater proportion in relation to certain preoperative conditions, type of surgery, and use of endotracheal intubation (25–27) (see Fig. 4.5).
Upper respiratory infections (URIs) remain a significant cause of perioperative respiratory morbidity in association with intubation of the trachea (28). Children with a URI are two to seven times more likely to experience respiratory-related adverse events (29). However, data suggest that while children with an acute, uncomplicated URI have an increased incidence of respiratory events during anesthesia, they have no increased morbidity; therefore, proceeding with a well-designed (possibly airway “sparing”) anesthetic is acceptable. On the other hand, signs and symptoms of lower respiratory tract infection warrant cancellation of the procedure (10,30).
CLINICAL PEARL Children with URIs have more frequent airway events, but they are usually minor and without sequelae. Clinical judgment and assessment of the severity of comorbidities can help to differentiate those who can proceed from those who should be canceled, and heightened vigilance is necessary in all cases.

Full access? Get Clinical Tree