CHAPTER 29
Renal Tumors
Luiz M. Kolankiewicz, MD, MC, Maj USAFR
Renal cell carcinomas (RCCs), either as benign or malignant renal tumors, have obscure signs and symptoms, are clinically occult, and often are diagnosed by primary care providers (PCPs) in the initial workup of a classical triad of flank pain, palpable abdominal renal mass, and hematuria. In addition, renal masses are nowadays more frequently seen in the evaluation of nonurologic abnormalities on sonograms, CT scanning, and MRI. Hematuria, gross or microscopic, is usually observed only if the tumor has invaded the collecting system; this occurs in only a small fraction of patients, but when present strongly suggests advanced disease. The PCP should be familiar with the appropriate evaluation and treatment of common renal neoplasms. Consultation with a urologist is usually necessary for proper evaluation, diagnosis, and treatment options.
This chapter concentrates on the epidemiology, pathology, clinical and radiographic presentation, staging methods, and surgical and systemic treatment modalities of primary renal neoplasms.
 OVERVIEW OF RENAL TUMORS
OVERVIEW OF RENAL TUMORS
Diagnostic Criteria
Although incidental detection of renal masses is the primary diagnostic modality, many are still found in the workup of hematuria.
Hematuria is defined as abnormal excretion of red blood cells (RBCs) into the urine. Healthy individuals excrete about 1 million RBCs per day in their urine, or about one to three RBCs per high-power field (HPF). Therefore, excretion of more than three RBCs per HPF is abnormal and may warrant further evaluation. Asymptomatic “microscopic hematuria” is very common. Microscopic hematuria may be detected in up to 13% of adults. Hematuria can be a sign of advanced underlying disease and should not be ignored.
Gross hematuria occurs when there is enough blood present in the urine to turn it red or brown. Microscopic and gross hematuria are generally caused by the same conditions; however, they may manifest differently for specific pathological conditions. Therefore, the diagnostic approaches to these two conditions are different. Routine screening of healthy individuals for the presence of hematuria is not recommended by the U.S. Preventive Services Task Force (USPSTF). Despite official recommendations, urinalyses are nonetheless frequently obtained as a screening test. Whenever a urinalysis reveals hematuria, the person’s age, gender, race, medical history, and physical findings should be considered in deciding whether to proceed with further evaluation.
History and Physical Examination
A complete history and physical examination are part of any hematuria workup. Three major factors influence the workup: the patient’s gender, race, and age. The common causes of hematuria in children and young adults are different from those in older individuals. Hematuria in adults older than 40 years must be considered a sign of malignancy (of the bladder, upper urinary tract, or kidney origin) until proved otherwise. Although malignancy is much less frequent in patients with hematuria who are younger than 40 years, a nephroblastoma (Wilms’ tumor) should be considered and the precise nature of the hematuria should be elucidated.
Hematuria accompanied by pain, either in the flank or the pelvis, may be an indication of stone disease. Colicky pain suggests ureteral obstruction from a stone, blood clot, or sloughed renal papilla. This is especially the case if the pain radiates to the testicle or labia. Fever may be an indication of an infection, possibly pyelonephritis. Pyuria is significant in almost all cases, and hematuria without pyuria should not be attributed to infection. The passage of blood clots can sometimes help to localize the site of bleeding to the upper tracts—long and string-like versus more clumped (bladder). Recent trauma may be readily apparent in some patients, but hematuria secondary to apparently trivial trauma may indicate a condition such as aureteropelvic junction obstruction. Recent strenuous physical exercise is sometimes associated with hematuria as well. A past history of urologic conditions or procedures is obviously important. A family history of urologic disease may be crucial because some cases of RCC are familial, with and without von Hippel–Lindau (VHL) disease. Numerous environmental and occupational exposures have been implicated in the etiology of RCC. These include tobacco use, obesity, and NSAID (nonsteroidal anti-inflamatory drug) use, as well as occupational exposure to toxic compounds such as cadmium and asbestos. Specific exposure to rubber and dye elements are particularly relevant to transitional cell carcinoma (TCC). Polycystic kidney disease, either acquired or congenital, will rarely also confer a propensity to development of renal cell cancer.
A thorough exam of the flank, abdomen, and genitalia should be included in the physical. Any unusual mass should be carefully characterized.
Diagnostic Studies
Urine excretion trajectory is exposed to the urothelium in the renal pelvis, ureters, bladder, and urethra. Urine can contain many molecular markers that could be associated with neoplasia. Urinary exfoliative cytology is important in the workup of hematuria. TCC, whether of the bladder or upper urinary tract, has a loosely coherent outer layer. These are the cells that are often identified by urinary cytology. Bladder cancers are much more common than renal tumors. Although a negative result on cytology tests does not rule out bladder cancer, a positive cytology result often helps direct the course of therapy. In addition, because some renal TCCs are difficult to distinguish from RCC, a positive cytology result can shift the treatment plan for renal tumors. As will be described later, the surgical approach for renal TCC is different from that for RCC.
Urine biomarkers have recently become available in the evaluation of hematuria. The Bard BTA-Stat/BTA-TRAK (bladder tumor antigen assay) is more sensitive than cytology but less specific. This test is particularly sensitive in detecting bladder cancer in patients with history of schistosomiasis (Miyanaga et al., 2003). The Matritech NMP22 (nuclear matrix protein) is used for surveillance rather than screening (Mattoli et al., 2003). Other biomarker tests undergoing experimental development include NMP52 (nuclear matrix protein 52), Cytokeartin 8 and 18, TRAP (telomerase repeat amplification protocol), Survivin (apoptosis inhibitor BioDot test), and Microsatellite analysis detecting alteration in bladder tumor cells by polymerase chain reaction. These tests identify unique markers in the urine of patients with bladder cancer (Loeshwar et al., 2005). Although they are experimental and their specific role has yet to be defined, these tests may be eventually helpful in following patients with bladder cancer. They are mentioned here for completeness in the diagnostic criteria for hematuria.
Computed tomography (CT) and ultrasonography (USG) are the mainstays of evaluation of a suspected renal mass. Intravenous urography (IVU), an invasive procedure of the past, remains useful in the evaluation of hematuria but is not routinely used anymore. USG is an excellent test to differentiate solid from cystic lesions of the kidney. However, USG alone is not sensitive enough to identify most urothelial tumors, specifically TCC of the renal pelvis or ureter. Masses seen on USG that meet the strict criteria for a simple cyst require no further evaluation. These criteria include a fluid-filled mass with a distinct posterior wall and acoustic enhancement, without calcification, septation, or nodularity. In some cases, USG may not confirm the existence of a mass. A CT scan should then be obtained with and without contrast to take advantage of the vascular nature of most RCCs. In patients allergic to contrast media and unwilling to accept intravenous contrast after steroid preparation, and in patients with marginal renal function, MRI with gadolinium is also an excellent test in the evaluation of renal masses.
MRI with gadolinium is not usually recommended for patients with existing chronic kidney disease (CKD) and/or in acute kidney injury (AKI) secondary to reports related to complication associated with development of nephrogenic systemic fibrosis (NSF). To date there is no effective therapy for NSF. Thus, avoidance of exposure by using alternative imaging modalities is the best option (Broome et al., 2007; Thomsen, 2009). Renal angiography is seldom necessary today, although in the past it was often used to clarify an abnormal IVU. Angiography is sometimes helpful in preparation for a partial nephrectomy, especially in a patient with a solitary kidney or multiple renal masses.
Imaging studies provide information about the kidneys and ureters but cannot adequately assess the bladder. Large bladder tumors are sometimes seen on an IVU or CT scan, but smaller tumors and carcinoma in situ are not reliably detected on any imaging test. Cystoscopy is necessary to complete the hematuria workup. This can often be performed in the urologist’s office or in an ambulatory surgery center. If the result of the urine cytology is positive, or if an abnormality is detected on upper-tract imaging studies, a cystoscopy may be performed with anesthesia. This allows samples to be taken for bladder biopsy or ureteropyelography to be accomplished without patient discomfort.
 SPECIFIC RENAL TUMORS
SPECIFIC RENAL TUMORS
Renal Adenomas
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
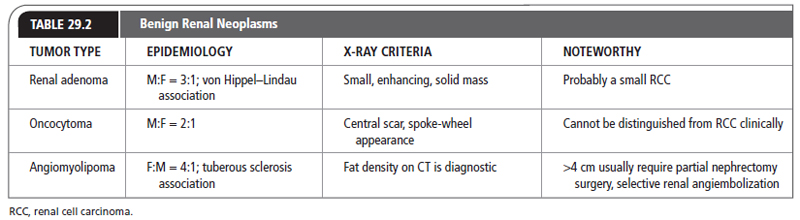
The classification of “renal adenoma” remains controversial in the urologic literature Table 29.2 presents a synopsis of the following discussion about specific renal tumors. It may be possible to distinguish an RCC from a renal adenoma based on nuclear and cytoplasmic criteria, but there are no reliable gross, microscopic, or ultrastructural differences between the two lesions. Previously, size alone was the standard criterion. This was based on the autopsy study by Bell (1938), demonstrating that renal lesions smaller than 3 cm rarely metastasized, whereas almost 70% of lesions >3 cm had metastasized. There are, however, several reports of lesions as small as 5 mm with metastases. Small, solid renal masses that enhance with intravenous contrast are probably small RCCs.
EPIDEMIOLOGY
In autopsy series, renal adenomas have been found in about 7% to 23% of cases (Licht, 1995). In large screening ultrasound series, however, the clinical detection rate is much <1%. The male/female ratio is 3:1. Certain diseases carry a significantly higher incidence of adenomas. Patients with VHL disease have a tendency to develop renal cysts and solid renal tumors. Some of these may be adenomas, but there is an association with more aggressive RCC as well. Patients with acquired renal cystic disease who are on dialysis for end-stage renal disease are also prone to develop small but potentially metastatic renal tumors. These have historically been labeled adenomas, but are probably small RCCs.
Treatment Options, Expected Outcomes, and Comprehensive Management
Most small adenomas are asymptomatic. They are detected incidentally on CT or USG during the workup for an unrelated medical problem. Rarely, they can present as a source of bleeding. Even larger adenomas are usually discovered incidentally, but they are more likely to present with symptoms referable to the urinary tract. Flank pain and hematuria have been associated with larger adenomas.
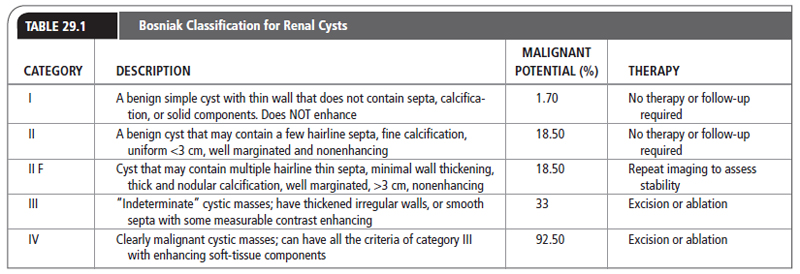
Renal adenomas appear as solid masses on imaging. They cannot be distinguished from RCC by imaging techniques, and most urologists think these potentially malignant tumors should undergo treatment as if they were RCCs. How should these small RCCs or renal adenomas be managed? Although the standard therapeutic approach for RCC remains radical nephrectomy, there is growing interest in the use of partial nephrectomy, especially for incidentally discovered lesions. In select cases, it may even be reasonable to observe small tumors. In general, Bosniak class I, II, and IIF cysts are likely to represent benign lesions, thus requiring either no therapy or just continued radiographic follow-up, in the case of class IIF lesions. These recommendations are based on a number of series published in the literature by Bosniak (2005). These series included both radiographic and pathologic follow-up. Bosniak showed that, for lesions <3 cm at diagnosis, and followed for at least 2 years, no metastases were clinically detectable. The majority of patients eventually went on to have surgery, and none developed metastases. This “watchful waiting” approach may be acceptable for an elderly patient with other significant medical problems, but it should be undertaken in consultation with a urologist. It is certainly not to be suggested for the young and otherwise healthy patient, because small renal tumors do grow and do have the capacity to metastasize. Table 29.1 lists the Bosniak classification of different renal cysts. Figure 29.1 shows radiographic examples of Bosniak cysts.
 RENAL ONCOCYTOMA
RENAL ONCOCYTOMA
Anatomy, Physiology, and Pathology
Renal oncocytoma is the most common benign tumor that appears as an enhancing renal mass on cross-sectional imaging; it is presumed to be RCC until surgical excision, representing challenges in preoperative diagnosis for the urologist. Grossly, oncocytomas are well circumscribed with pseudocapsule tumors and a characteristic mahogany color. A large stellate central scar is often seen. Hemorrhage and necrosis are typically absent. Microscopically, the tumor is composed of large well-differentiated cells with intensely eosinophilic cytoplasm containing abundant mitochondria. These oncocytes rarely exhibit mitoses.
FIGURE 29.1
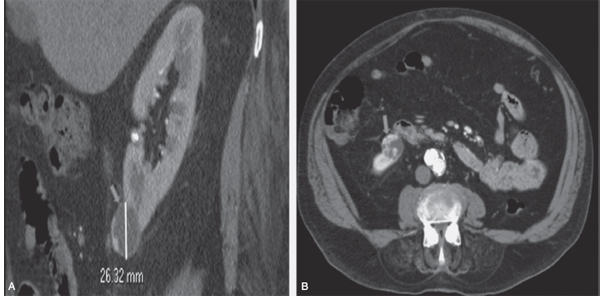
(A) CT scan of a Bosniak III renal cyst with a slightly thickened, nonuniform, slightly irregular enhancing cystic mass. The cystic mass is <3 cm. (B) CTA scan showing progression of lesion from A: now a Bosniak IV lesion with irregular thick septations, a visible enhancing wall, coarse calcifications, and areas of soft-tissue enhancement. The cystic mass is >3 cm.
Source: Courtesy of Dr. Lien Burn.
Epidemiology
Oncocytomas account for 3% to 7% of all renal tumors (Adamy et al., 2011). The male/female ratio is 2:1. They are usually solitary, and the peak age of incidence is 55 years. They are occasionally found in the same kidney with RCC, on rare occasions within the same lesion.
Treatment Options, Expected Outcomes, and Comprehensive Management
Approximately 70% of oncocytomas are detected incidentally. The remainder present with complaints referable to the genitourinary system. Hematuria, flank pain, and a flank mass are the typical symptoms. Oncocytomas appear as solid masses and present the greatest difficulty for the clinician in confidently distinguishing them from RCC on clinical and radiologic grounds. A central scar seen on CT or a “spoke-wheel” appearance on angiography may suggest an oncocytoma, but there is simply no reliable method to rule out RCC preoperatively.
Pure oncocytomas are benign lesions. However, because there is no reliable clinical method to differentiate oncocytomas from RCCs, they are usually treated as RCCs and managed with nephrectomy. As is the case for renal adenomas, small, incidentally discovered lesions suggestive of oncocytoma may be treated successfully with a partial nephrectomy. Because of the occasional occurrence of oncocytoma and RCC in the same lesion, and because some RCCs have oncocytic features, the diagnosis of oncocytoma cannot be made with a small sampling of tissue. However, the diagnostic accuracy of percutaneous biopsy has improved, especially when a core biopsy is done in addition to a fine-needle aspiration and the concomitant use of immunostains, prompting some investigators to revisit the role of the biopsy in the management of some patients with an incidental renal tumor (Schmidbauer et al., 2008).
 ANGIOMYOLIPOMA
ANGIOMYOLIPOMA
Anatomy, Physiology, and Pathology
Angiomyolipoma is now considered to be of neural crest origin and possibly derived from perivascular epithelial cells rather than (as initially postulated) a form of hamartoma and or choristoma. The gross appearance is determined by the relative amounts of the various cellular components. If the lesion is composed predominantly of adipose tissue, it will have a homogeneous yellowish appearance, but all three cell types must be present to establish the diagnosis. The lesion will be more heterogeneous if there is an even distribution of fat, muscle, and vessels. Calcification and necrosis are rare, but hemorrhage is frequent.
Epidemiology
Angiomyolipoma accounts for <10% of renal tumors (Eble, Togashi, & Pisani, 2004; Goyal, Gersbach, Yang, & Rohan, 2013). It is a benign neoplasm consisting of thick-walled aneurismal vessels, smooth muscle, and adipose tissue that can be reliably diagnosed by cross-section radiographic imaging.
Angiomyolipomas are seen in two distinct clinical settings: sporadic and in association with tuberous sclerosis. The sporadic form accounts for more than 80% of cases. It is more common in women, with a female/male ratio of 4:1, and the mean age is 43 years (Eble et al., 2004; Goyal et al., 2013). Tuberous sclerosis is a congenital and familial disorder characterized by brain gliosis, mental retardation, epilepsy, adenoma sebaceum, and hamartomas of the retina, lungs, liver, pancreas, bone, and kidneys. Angiomyolipomas associated with tuberous sclerosis present as multiple, bilateral, larger lesions, at a younger age, and are more likely to be symptomatic and require surgical intervention.
Treatment Options, Expected Outcome, and Comprehensive Management
Plain radiographs occasionally show lucent areas within large angiomyolipomas, suggesting the diagnosis, but typically the IVU demonstrates an expansile mass that cannot be distinguished from RCC. On USG, they are usually more echogenic than surrounding parenchyma. They share this feature with about one third of RCCs. However, with the development of high-quality CT scanning, it was realized that the detection of fat within a renal lesion allows confident preoperative diagnosis and the potential to avoid surgery in most cases that are not symptomatic. Table 29.2 illustrates this information.
In fat-poor suspected angiomyolipoma, a percutaneous biopsy can provide a diagnosis with the advent of HMB-45 (monoclonal antibody against melanoma-associated antigen; Catalano, Samir, Sahani, & Hahn, 2008; He et al., 2011).
The most common presentation is incidental discovery during imaging for other medical conditions. Patients may present with acute flank pain with or without spontaneous hemorrhage. Occasionally, the onset of symptoms is preceded by seemingly trivial trauma. The blood vessels of angiomyolipoma lack a complete elastic layer, predisposing these lesions to aneurysm formation and bleeding. Steiner et al. demonstrated that lesions <4 cm rarely become symptomatic, whereas lesions >4 cm require surgical intervention in about half the cases for bleeding (Steiner, Goldman, Fishman, & Marshall, 1993). Treatment must be individualized, based on presentation, pregnancy, tumor size, and renal function. In case of acute hemorrhage, renal angioembolization is the treatment of choice.
When the CT scan is unequivocal and the patient is asymptomatic, no intervention is required for small angiomyolipomas. In cases where the diagnosis is in question, exploration and partial or radical nephrectomy may be necessary. Symptomatic patients with angiomyolipomas can often be treated successfully with percutaneous angioembolization.
Malignant Renal Neoplasms
RENAL CELL CARCINOMA
Anatomy, Physiology, and Pathology • The origin of RCC is the renal cortex, and these tumors occur with equal frequency in the right and left kidneys. Grossly, the tumors are often yellow or orange because of the abundance of fat seen in the clear-cell variant. The clear cytoplasm observed by light microscopy of the clear-cell variety is caused by removal of glycogen and lipids during histologic processing. The granular type is usually more white to gray. The sarcomatoid cell type is less frequently seen. Usually, there is a mixed picture. As RCC grows, it compresses the normal parenchyma, forming a pseudocapsule. RCC has a propensity for invasion into vascular spaces, including the renal vein, inferior vena cava, and occasionally even the right atrium.
Histologic grading of RCC cells has been used with variable success to predict the clinical behavior of these tumors. Compared to pathologic stage, no additional prognostic information has been consistently obtained.
Epidemiology • RCC accounts for 85% of all renal parenchymal cancers and represents 2% to 3% of all adult malignancies. RCC has been known by many different terms: historically, hypernephroma, Grawitz’s tumor in 1883, and later nephrocarcinoma. There were approximately 58,000 new cases in 2009, resulting in 13,000 deaths (Jemal et al., 2009). It occurs most commonly in the fifth to sixth decade, although it has been reported in children and young adults. The male/female ratio is 2:1. Recent advances in imaging have led to increased incidental detection of RCC and improved prognosis of patients with such tumors.
Most cases of RCC are sporadic in nature. However, it has been shown that in rare familial cases and in RCC associated with VHL disease, loss of tumor suppressor gene activity, particularly at the VHL locus on chromosome 3p, may be responsible for tumorigenesis (Franklin, Figlin, & Belldegrun, 1996). Other rare genetic disorder associations include hereditary papillary renal cancer, hereditary leiomyoma renal cancer syndrome, and Birt–Hogg–Dube syndrome (an autosomal-dominant genetic disorder that involves susceptibility to renal cancer, renal and pulmonary cysts, and noncancerous tumors of the hair follicles).
The ways environmental and genetic factors interact in the pathogenesis of RCC have not been completely elucidated. Two strong cases for environmental exposure are cigarette smoking and phenacetin-containing analgesic abuse. There is a dose-related risk of developing RCC in cigarette smokers (approximately a twofold increase) and contributes to as many as one third of all reported cases. An increased risk has also been reported in shoe workers, leather tanners, and workers exposed to cadmium, petroleum products, and asbestos.
Treatment Options, Expected Outcomes, and Comprehensive Management
The classic triad of hematuria, pain, and palpable flank mass occurs in only 10% of cases, but when present strongly suggest locally advanced disease. More commonly, a patient will present with one or two of these symptoms. Other symptoms may be associated with paraneoplastic syndromes from elaboration of various humoral factors. In some instances, this may be due to ectopic production of various hormones (e.g., erythopoetin, parathyroid-related protein, human chorionic gonadotropins, adrenalcortical tropic hormone (ACTH)-like substance, renin, glucagon, insulin, and other hormones). Stauffer’s syndrome (abnormal liver enzymes), hypercalcemia, polycythemia, and hypertension are the best known of these (Gold, Fefer, & Thompson, 1996). These findings do not imply metastatic disease.
The most common test to evaluate extent of disease is the CT scan (Figure 29.2). RCC is seen as a solid lesion that enhances after intravenous contrast administration. An increase in Hounsfield units of at least 20 is considered positive enhancement. Ten to 20 units of enhancement are considered intermediate and inconclusive. Approximately 10% of RCCs are hypovascular, however, and will not enhance with intravenous contrast (Gillenwater, Grayhack, Howards, & Duckett, 1996).
When involvement of the vena cava by tumor thrombus is in question, an MRI is often helpful and superior to CT with contrast. Caution should be exercised if MRI-gadolinium is needed, especially in patients with existing CKD, regarding the development of a rare but often serious syndrome known as NSF or nephrogenic fibrosing dermopathy that involves fibrosis of skin, joints, eyes, and internal organs. This syndrome was first identified in 1997 and to date is not fully understood (Thomsen, 2009). Occasionally, a cavogram or transesophageal USG is necessary to determine the exact location of a tumor thrombus in the vena cava. Knowledge of the superior extent of the thrombus is crucial for planning the surgical approach. Even patients with tumor in the right atrium can be cured of RCC with a radical nephrectomy and thrombectomy, although the potential morbidity and mortality rates of the procedure are certainly higher in this setting.
Tumor stage is recognized as the most important prognostic factor in RCC (Table 29.3). The TNM classification has supplanted the previously used Robson system. The TNM system was modified in 2002 with the reclassification of T1 tumors into T1a tumor <4 cm and T1b tumor >4 cm. It also included renal sinus invasion in the T3a classification and renal vein invasion. The seventh edition of the American Joint Cancer Classification (AJCC), released in 2010, contains additional relevant changes to T3 and T4 definitions as well as to node positive disease (N1) regardless of number of positive nodes (Edge et al., 2010).
Nuclear grade (Fuhrman classification) has recently become a more accepted prognostic factor for clear-cell renal carcinoma. The transition from Robson, TNM staging, and Fuhrman grade to integrate multiple validated prognostic factors from Memorial Sloan-Kettering Cancer Center or “MSKCC criteria” has been validated across different institutions. These criteria are currently the most commonly used nomogram that combine tumor stage, tumor size, histological subtype, Fuhrman nuclear grade, necrosis, vascular invasion, and symptoms predicting the probability of freedom from recurrence at 5 years after treatment (Table 29.4).
Table 29.3 provides the stage group classification of RCC. Table 29.4 compares information pertinent to tumor size and staging between previous classifications.
Surgical excision remains the standard therapy for clinically organ-confined RCC. Radical nephrectomy, which involves the early ligation of the renal artery and renal vein, entails en bloc removal of the kidney and its enveloping fascia. The classic radical nephrectomy includes the ipsilateral removal of adrenal gland as well. However, recent studies have shown a very low, <5% involvement of the adrenal gland (Robey & Schellhammer, 1986). Thus, adrenalectomy has fallen out of favor for all but large upper pole lesions, which may be more likely to involve the adrenal by direct extension. Regional lymphadenectomy is often performed, but there are no controlled studies that demonstrate improved survival with this additional procedure (Hellenthal et al., 2009).
The main value of removing the lymph nodes may be in more accurate staging, and nowadays multiple preoperative and intraoperative evaluative nomograms exist to assist the surgeon in deciding the benefit of lymph node dissection. Partial nephrectomy or nephron-sparing surgery implies removal of the tumor while leaving enough functional renal parenchyma to support life without the need for dialysis in the event that the other kidney is already absent or lost in the future. For this reason, the role of nephron-sparing surgery was expanded from elective to more standard of care. A partial nephrectomy is also often performed in a patient with impaired renal function or a patient with bilateral renal tumors. The size and location of the tumor are crucial determinants of whether a partial nephrectomy can be attempted. Because of the increase in the number of smaller, incidentally discovered renal masses, interest has grown in the use of partial nephrectomy even in patients with normal renal function and a normal contralateral kidney. Results using this approach in a series by Herr (1994) were excellent, with survival of about 95% and acceptable local recurrence rates of about 2%.
FIGURE 29.2
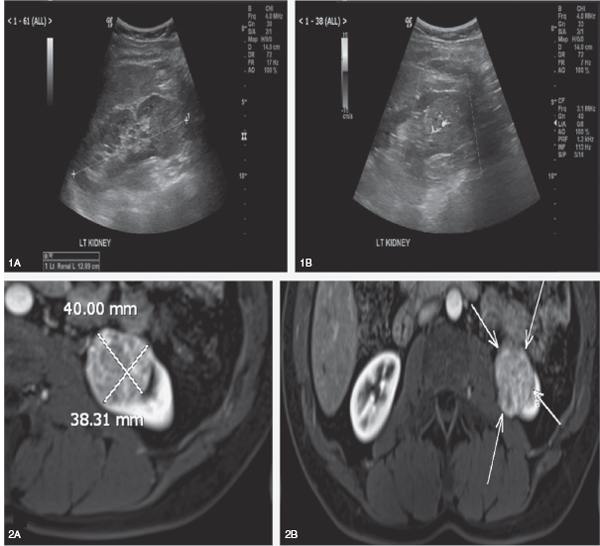
(1A–B) Doppler ultrasound scan shows a well-circumscribed left lower renal pole solid mass with internal vascularity. (2A–B) Abdomen MRI shows heterogeneously enhancing >3 cm solid mass in left lower renal pole (arrows).
Source: Courtesy of Dr. Lien Burn.

Full access? Get Clinical Tree