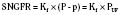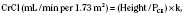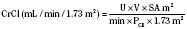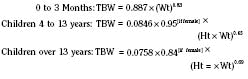CHAPTER 5 Regulation of Fluids and Electrolytes
Overview of anatomy and physiology
Renal Blood Flow
Despite accounting for only 0.5% of body weight, the kidneys receive about 25% of the cardiac output, with a blood flow of approximately 4 mL/min per gram of kidney tissue. Renal plasma flow (RPF) in women is slightly lower than it is in men, even when normalized for body surface area, averaging 592 ± 153 mL/min per 1.73 m2 and 654 ± 163 mL/min per 1.73 m2, respectively (Smith, 1943). In children between the ages of 6 months and 1 year, normalized RPF is half that of adults but increases progressively to reach adult levels at about 3 years of age (McCrory, 1972). After the age of 30 years, renal blood flow (RBF) decreases progressively; by the age of 90 years, it is approximately half of the value present at 20 years (Davies and Shock, 1950). This generous supply provides not only for the basal metabolic needs of the kidneys but also for the high demands of ultrafiltration.
The basic arterial supply of the kidneys is a single renal artery that divides into large anterior and posterior branches and subsequently into segmental or interlobar arteries. The latter form the arcuate and interlobular arteries. These blood vessels are end-arteries and therefore predisposed to tissue infarction in the presence of emboli. The arcuate arteries are short, large-caliber vessels that supply blood to the afferent arterioles of the glomeruli at a mean pressure of 45 mm Hg, which is higher than that found in most capillary beds. This high hydraulic pressure and large endothelial pore size lead to enhanced glomerular filtration (Brenner and Beeuwkes, 1978).
Ninety percent of RBF goes to the cortex, which accounts for 75% of the renal weight, whereas the medulla and the rest of the kidneys receive 25% of the RBF. Although cortical blood flow is 5 to 6 mL/g per minute, outer medullary blood flow decreases to 1.3 to 2.3 mL/g per minute, and the flow to the papilla is as low as 0.22 to 0.42 mL/g per minute (Dorkin and Brenner, 1991). The unevenness in the distribution of RBF between the cortex and the medulla is necessary to develop and maintain the medullary gradient of osmotically active solutes that drive the countercurrent exchange/multiplier, which is essential for the elaboration of concentrated urine. Outer medullary blood flow may preferentially supply the loop of Henle, thereby accounting for the striking influence of loop diuretics in that region. Furthermore, papillary blood flow is far greater than the metabolic needs of the renal parenchyma and is well adapted to the countercurrent concentrating mechanism characteristic of this region.
RBF remains almost constant over a range of systolic blood pressures from 80 to 180 mm Hg, a phenomenon known as autoregulation. Consequently, glomerular filtration is also constant over this range of pressures as a result of adaptations in the renal vascular resistance (Selkurt et al., 1949, Gertz et al., 1966). Because the changes in resistance that accompany graded reductions in renal perfusion pressure occur in both denervated and isolated perfused kidneys, autoregulation appears not to depend on extrinsic neural or hormonal factors (Thurau, 1964). According to the “myogenic hypothesis” first proposed by Bayliss (1902), the stimulus for vascular smooth muscle contraction in response to increasing intraluminal pressure is either the transmural pressure itself or the increase in the tension of the vascular wall. An increase in perfusion pressure, which initially distends the vascular wall, is followed by a contraction of the resistance vessels and a return of blood flow to basal levels.
There are only a few studies of autoregulation of RBF in developing animals. Aortic constriction in adult animals reduces renal perfusion by 30% but has minimal effects on RBF and glomerular filtration rate, compared with the significant changes observed in 4- to 5-week-old rats (Yared and Yoskioka, 1989). Furthermore, it has been demonstrated that autoregulation of RBF in young rats occurs at renal perfusion pressures between 70 and 100 mm Hg, compared with pressures of 100 to 130 mm Hg in adult rats (Chevalier and Kaiser, 1985). A similar increase in the pressure set point for autoregulation has been found in dogs (Jose et al., 1975). It appears that autoregulation of RBF occurs in the very young and is sufficient to maintain blood flow constant over a wide range of perfusion pressures that are physiologically adequate for the age. No such human studies are available.
Several substances have been proposed to participate in the autoregulation of RBF, including vasoconstrictor and vasodilator prostaglandins, kinins, adenosine, vasopressin, the renin-angiotensin-aldosterone system, endothelin, and endopeptidases (Herbacznska-Cedro and Vane, 1973, Osswald et al., 1978, Maier et al., 1981, Schnermann et al., 1984). Nitric oxide (NO), previously known as endothelium-derived relaxing factor (EDRF), has also been shown to play an important role in regulating renal vascular tone through its vasodilatory action. Bradykinin, thrombin, histamine, serotonin, and acetylcholine act on endothelial receptors to activate phospholipase C, which in turn results in the formation of inositol triphosphate and diacylglycerol, resulting in the release of intracellular calcium (Marsden and Brenner, 1991, Luscher et al., 1992). This in turn stimulates the synthesis of NO from L-arginine. Other factors that stimulate the formation of NO include hypoxia, calcium ionophores, and mechanical stimuli to the endothelium. NO increases RBF by decreasing efferent arteriolar vascular resistance, while glomerular filtration remains unchanged (Marsden and Brenner, 1991).
Because in mature kidneys, autoregulation is lost at arterial pressures less than 80 mm Hg, the lower physiologic pressures prevailing in the newborn period may be expected to limit this important control mechanism. There is evidence both to support and to refute this conclusion (Kleinman and Lubbe, 1972, Jose et al., 1975).
Renal Physiology
The route by which water and other solutes are filtered from the blood is not fully understood, but it appears that plasma ultrafiltrate traverses the large fenestrations of the glomerular capillary endothelium and penetrates the basement membrane and the slit pores located between the podocyte foot processes. Filtration of large molecules is greatly influenced by the size and charge of the specific molecule, as well as by the integrity and charge of the glomerular basement membrane. Abnormalities in various structural proteins of the slit-pore diaphragm such as nephrin, podocin, and α-actinin may be responsible for several proteinuric disorders (Mundel and Shankland, 2002). In general, the endothelium and the lamina rara interna of the glomerular basement membrane slow the filtration of circulating polyanions such as albumin (Ryan and Karnovsky, 1976). The lamina rara externa and the slit pores slow the filtration of cationic macromolecules such as lactoperoxidase (Graham and Kellermeyer, 1968). Neutral polymers such as ferritin are not filtered because of their large molecular size and shape (Farauhar et al., 1961). Molecules with a radius of 4.2 nm or more are excluded from the glomerular filtrate. In practical terms, red cells, white cells, platelets, and most proteins are restricted to the circulation.
Glomerular Filtration
Among the main functions performed by the kidneys is the process of glomerular filtration. The glomerulus is primarily responsible for the filtration of plasma. The glomerular filtration rate (GFR) is the product of the filtration rate in a single nephron and the number of such nephrons, which range from 0.7 to 1.4 million in each kidney (Keller et al., 2003). Clearance, which is defined as the volume of plasma cleared of a substance within a given time, provides only an estimate or approximation of GFR.
Glomerular filtration is driven by hydrostatic pressure, which forces water and small solutes across the filtration barrier. In healthy individuals, changes in hydrostatic pressure rarely affect single-nephron GFR because autoregulatory mechanisms sustain or maintain a constant glomerular capillary pressure over a large range of systemic blood pressure (Robertson et al., 1972). Hydrostatic pressure is opposed by the oncotic pressure produced by plasma proteins and the hydrostatic pressure within Bowman’s capsule. Mathematically, this relation can be expressed by the following equation:
SNGFR is the single-nephron glomerular filtration rate; Kf is the glomerular ultrafiltration coefficient; P and p are the average hydraulic and osmotic pressure differences, respectively; and PUF is the net ultrafiltration pressure. As plasma water is filtered, the proteins within the capillaries become more concentrated, so oncotic pressure increases at the distal end of the glomerular capillary loop and the rate of filtration ceases at the efferent capillary (Blantz, 1977). Under normal conditions, about 20% of the plasma water that enters the glomerular capillary bed is filtered; this quantity is referred to as the filtration fraction
Serum-creatinine concentrations vary by age and gender. In 1-year-old girls values are 0.35 ± 0.05 mg/dL (mean ± SD) and rise gradually to 0.7 ± 0.02 mg/dL (mean ± SD) by 17 years of age; boys have corresponding mean values that are 0.05 mg/dL higher until 15 years of age and 0.1 mg/dL higher subsequently (Schwartz et al., 1987). Expected creatinine-excretion rates in 24-hour urine collections are often used to validate such collections. Values range from 8 to 14 mg/kg per day in neonates and in infants younger than 1 year of age, with an increase to about 22 ± 7 mg/kg per day (mean ± SD) in preadolescent children of either gender (Hellerstein et al., 2001). Subsequently, creatinine excretion in boys is 27 ± 3.4 mg/kg per day.
where height is in centimeters, PCR is the plasma-creatinine concentration in mg/dL, and k is a constant proportion to muscle mass. The value of k is 0.45 in full-term newborns and until 1 year of age, 0.55 in children 2 years of age and older and in adolescent girls, and 0.70 in adolescent boys (Schwartz et al., 1987). Normal CrCl ranges from 90 to 143 mL/min per 1.73 m2, with a mean of 120 mL/min per 1.73 m2
In children with impaired renal function, GFR estimates based on creatinine methods may grossly overestimate the true GFR, because tubular and gastrointestinal secretion of creatinine increases disproportionately. Hence, serum creatinine concentrations are less reflective of filtration at the glomerulus. For example, Schwartz’s formulas overestimate GFR by 10% ± 3% when GFR is greater than 50 mL/min per 1.73 m2 but by 90% ± 15% when GFR is less than 50 mL/min per 1.73 m2. Other limitations of creatinine-based GFR determinations stem from variations of analytical assays, reference values ranging from 0.1 to 0.6 mg/dL in children younger than 9 years of age, diurnal variation in serum creatinine levels resulting from high intake of cooked meat or intense exercise, influence of body mass index, and inaccurate urine collections—all of which make comparisons of GFR difficult over time, especially in growing children (Levey et al., 1988). Using cimetidine to block tubular secretion of creatinine before measuring CrCl in urine collections may improve such measurements (Hellerstein et al., 1998).
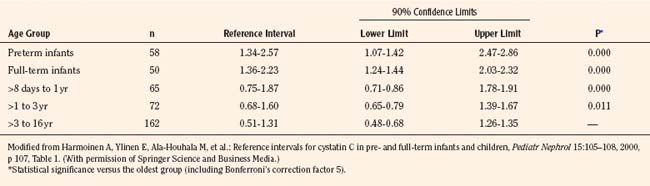
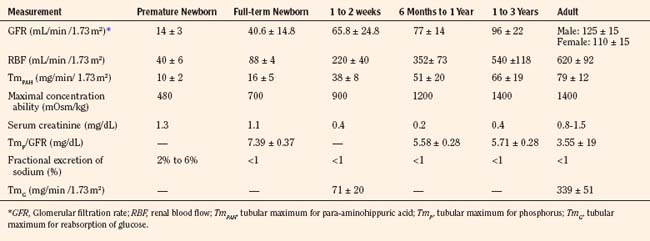
Measurement of cystatin-C, a 13-kDa serine proteinase produced at a constant rate by all nucleated cells, is purported to be a superior endogenous marker of filtration, because cystatin-C is less susceptible to variation than is plasma creatinine. A meta-analysis compared the correlation between GFR measured by inulin clearance, radiolabeled methods, nonlabeled iothalamate or iohexol, and either plasma creatinine or cystatin-C concentrations measured nephelometrically (Dharnidharka et al., 2002).The correlation between GFR and cystatin-C was significantly higher compared with plasma creatinine (0.846 versus 0.742, P < 0.001). Thus, cystatin-C measurements are becoming increasingly popular in clinical practice, and reference ranges have been generated in children up to 16 years of age (Table 5-1) (Bokenkamp et al., 1998; Finney et al., 2000; Harmoinen et al., 2000).
Studies in renal transplant donors and in individuals with various renal disorders have shown that plasma-creatinine concentration changes minimally as GFR falls to about 50 mL/min per 1.73 m2 (Fig. 5-1) (Shemesh, 1985). This compensation is largely the result of hypertrophy and hyperfiltration of the remaining nephrons. When more than 50% of the nephrons cease to function and “renal reserve” is outstripped, serum creatinine may rise rapidly in a parabolic fashion (Fig. 5-1). Thus, when a more accurate clinical assessment of GFR is desirable for research purposes, radiolabeled methods with an identity exceeding 97% give a better approximation of GFR relative to inulin clearance and may be more useful in aiding clinical decisions. In multicenter investigations conducted in the United States using a uniform method for GFR measurement, 125I-iothalamate is often used because this isotope has low radiation exposure and long isotope half-life and can be assayed at a central laboratory (Bajaj et al., 1996). Otherwise, 99mTc-diethylenetriaminepenta-acetic acid (Tc-DTPA) is commonly used to estimate GFR for routine clinical purposes. In other countries, 51Cr-ethylenediaminetetra-acetic acid (Cr-EDTA), which delivers a greater radiation dosage, is also popular, as are nonlabeled iothalamate and iohexol methods.
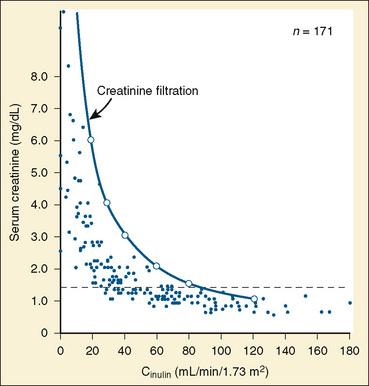
FIGURE 5-1 Relationship of serum creatinine to GFR.
(From Shemesh O, Golbetz H, Kriss JP, et al.: Limitation of creatinine as a filtration marker in glomerulopathic patients, Kidney Int 28:830, 1985.)
Although GFR may fluctuate, the kidneys retain the ability to regulate the rate of solute and water excretion according to changes in intake. This regulation is achieved by changes in tubular reabsorption rates—a phenomenon known as glomerular-tubular balance (Tucker and Blantz, 1977). The end result is preservation of ECF volume and chemical composition. Glomerular-tubular balance can be disturbed by several factors, including volume expansion, loop diuretics, and inappropriate secretion of antidiuretic hormone (ADH).
Overview of Tubular Function
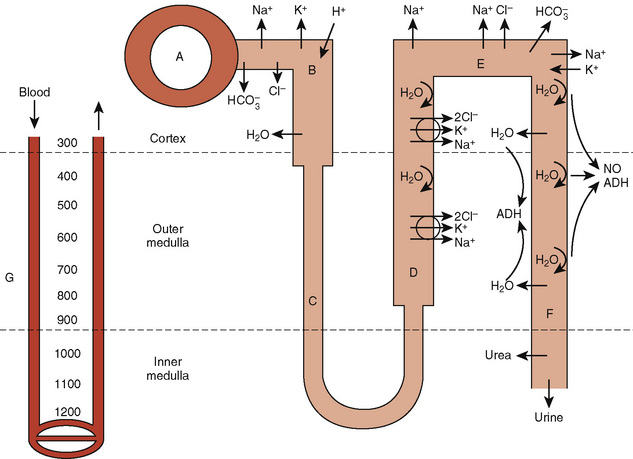
The proximal tubule is the site of reabsorption of large quantities of solute and filtered fluid (Fig. 5-2). Many transporters subserving tubular electrolyte transport have been characterized at the genetic level, and various pathologic disorders have been elucidated (Epstein, 1999). Under physiologic conditions, the proximal convoluted tubule isotonically reabsorbs 50% to 60% of the glomerular filtrate (Berry and Rector, 1991). The initial portion of the proximal convoluted tubule reabsorbs most of the filtered glucose, amino acids, and bicarbonate. Glucose and amino acids are absorbed actively, whereby they are transported against their electrochemical gradient, coupled to sodium (Na+). Active Na+ transport at the peritubular membrane provides the driving force that ultimately is responsible for other transport processes. The system is driven by sodium, Na+, K+, (activated) adenosine triphosphatase (Na+–, K+-ATPase), or the Na+ “pump,” which requires the presence of K+ in the peritubular fluid and is inhibited by ouabain.Micropuncture studies show that around 50% to 70% of the filtered Na+ is reabsorbed in this segment, mostly by a process of active cotransport.
The major fraction of filtered bicarbonate (HCO3−) is absorbed early in the proximal convoluted tubule. Hydrogen (H+) gains access to luminal fluid via an Na+/H+ electroneutral exchange mechanism and forms carbonic acid. The latter is dehydrated to H2O and CO2 under the influence of carbonic anhydrase. CO2 diffuses into the cell, and HCO3− is re-formed and ultimately absorbed into the bloodstream. In general, the concentration of HCO3− is maintained at 26 mmol/L, which is slightly below the renal threshold of approximately 28 mmol/L (Pitts and Lotspeich, 1946).
The renal clearance of glucose is exceedingly low, even after complete maturation of glomerular filtration. The amount filtered increases linearly as plasma glucose increases. Initially, all filtered glucose is reabsorbed until the renal threshold has been exceeded (at around 180 mg/dL), at which point filtered glucose appears in the urine. However, maximal tubular glucose (TmG) reabsorption is attained at a filtrate glucose concentration of about 350 mg/mL (Pitts, 1974). The reabsorption of glucose in the proximal tubule occurs via a carrier-mediated, Na+/glucose cotransport process across the apical membrane, followed by passive facilitated diffusion and active Na+ extrusion across the basolateral membrane.
The loop of Henle makes the formation of concentrated urine possible and contributes to the formation of dilute urine (Kokko, 1979). This dual function is achieved through the unique membrane properties of the loop, the postglomerular capillaries, and the hypertonicity of the interstitium. The proximity of the descending and ascending portions of loop allows it to function as a countercurrent multiplier, whereas the capillaries serve as countercurrent exchangers (Fig. 5-2). The descending loop of Henle abstracts water from tubular fluid, increasing the intraluminal concentrations of NaCl and other solutes. However, the intraluminal osmolality remains in equilibrium with the interstitium, where 50% of the osmolality results from urea. In the thin ascending limb of the loop of Henle, there is passive efflux of NaCl and urea into the interstitium. The thick ascending limb of the loop of Henle, by being impermeable to water, contributes to the formation of dilute urine.
The Kidneys and Antidiuretic Hormone
ADH plays a pivotal role in water homeostasis by acting on the most distal portion of the nephron. ADH is a cyclic octapeptide that, along with its carrier protein, neurophysin, is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus (Zimmerman and Defendini, 1977). The prohormone migrates along the nerve axons to the posterior pituitary gland, where it is stored as arginine vasopressin. It is released through exocytosis (Douglas, 1973).
Several variables affect ADH secretion. Physiologically, the most important factor is plasma osmolality. A very small rise in plasma osmolality is sufficient to trigger a response from the sensitive osmoreceptors located in and around the hypothalamic nuclei, leading to ADH secretion. Conversely, plasma ADH concentrations are less than 1 pg/mL at a physiologic plasma osmolality of less than 280 mOsm/kg water. The antidiuretic activity of ADH is maximal at plasma osmolality of greater than 295 mOsm/kg water, when plasma ADH exceeds 5 pg/mL (Robertson, 2001). Once plasma osmolality exceeds this limit—thus surpassing the capacity of the ADH system to affect maximal fluid retention—the organism depends on thirst to defend against dehydration. Intracerebral synthesis of angiotensin II largely mediates this thirst response and the oropharyngeal reflex. Atrial natriuretic peptide (ANP) opposes the release of ADH and of angiotensin II. In summary, plasma osmolality and Na+ are maintained within a narrow range. The upper limit of this range is determined by the sensitivity of the thirst mechanism located in the hypothalamus, whereas its lower range is affected by ADH release.
Nonhypovolemic conditions that stimulate ADH release often result in diminished urine volume, hyponatremia, fractional excretion of uric acid greater than 10%, low serum uric acid level (<4 mg/dL), and urinary sodium greater than 20 mEq/L (Albanese et al., 2001). These conditions result in hyponatremia. Conversely, inhibitors of ADH release or primary or acquired nephropathies may result in the inability to respond to ADH or to conserve water, and these inhibitors are often accompanied by polyuria with Uosm of less than 150 mOsm/kg, dehydration, and hypernatremia.
ADH has a major effect on the medullary thick ascending limb and thereby influences the countercurrent multiplier mechanism and urinary concentration. More directly, ADH binds to V2 receptors in the basolateral membrane of the collecting duct, causing the activation of adenylate cyclase and the formation of cyclic 3′,5′-adenosine monophosphate (cAMP) (Dorisa and Valtin, 1976; Schwartz et al., 1974). This results in insertion of aquaporin-2 water channels in apical membranes and in the activation of apical Na+ channels, which causes water conservation (Andreoli, 2001). These effects are counterbalanced by prostaglandin E2 (PGE2) and the calcium-sensing receptor in cells of the medullary thick ascending limb that mediate saluresis and diuresis.
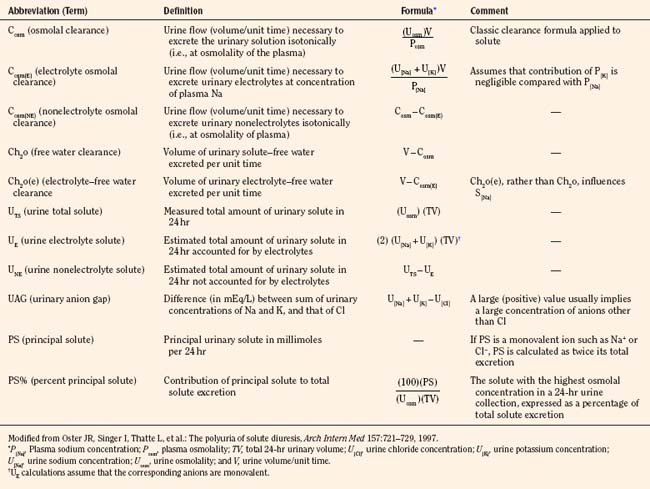
Polyuric syndromes can be separated on the basis of urine osmolality and generally consist of water diuresis, solute diuresis, or a mixed water-solute diuresis with typical Uosm of less than 150 mOsm/kg, 300 to 500 mOsm/kg, and 150 to 300 mOsm/kg, respectively (Oster et al., 1997). The etiology of polyuria may be facilitated by obtaining a urinalysis; a measurement of urine pH; and measurements of electrolytes, creatinine, osmolality, glucose, urea nitrogen, and bicarbonate, preferably in a timed urine collection together with the corresponding serum values. Such assessment may serve to prevent dehydration, acid-base disturbances, hypokalemia, or hypernatremia, which often accompany such polyuric disorders (Table 5-2) (Oster et al., 1997). Proper correction of acute hypernatremia is needed to prevent brain demyelination. Normal saline infusion may be the agent of choice in polyuric conditions associated with solute diuresis, whereas ADH and electrolyte-free fluid administration may be appropriate in cases of “pure” water diuresis. The recommended rate of correction of hypernatremia is about 10 mEq/L per 24 hours, amounting to a fall in plasma osmolality of about 20 mOsm/kg H2O per day (Adrogue and Madias, 2000b).
Renin-Angiotensin-Aldosterone System
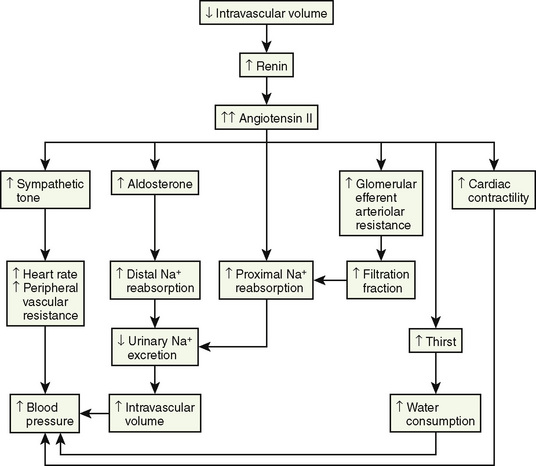
The renin-angiotensin-aldosterone axis plays a key role in control of vascular tone, Na+ and K+ homeostasis, and, ultimately, circulatory volume and cardiovascular and renal function. Renin is an enzyme with a molecular weight of 40 kDa that is synthesized and stored in the juxtaglomerular apparatus surrounding the afferent arterioles of the glomeruli (Davis and Freeman, 1976). The primary stimuli for renal renin release are reductions in renal-perfusion pressure, Na+ restriction, and Na+ loss as detected by the specialized macula densa cells located in the distal tubule. Mechanical (stretch of the afferent glomerular arterioles), neural (sympathetic nervous system), and hormonal (PGE2 and prostacyclin) stimuli act in an integrated fashion to regulate the rate of renin secretion (Fig. 5-3).
Once released into the circulation, renin cleaves the leucine-valine bond of angiotensinogen, forming angiotensin I. Angiotensin-converting enzyme that is present in the lungs, as well as in the kidneys, large caliber vessels, and other tissues, cleaves the carboxyl terminal (histidine-leucine dipeptide) from angiotensin I to form the biologically active angiotensin II (Ng and Vane, 1967).
Angiotensin II has numerous important hemodynamic functions that are mediated largely by binding to angiotensin-II T1-receptors in endothelial cells, tubular epithelial cells, and smooth muscle (Box 5-1) (Burnier and Brunner, 2000). It plays a key role in regulating blood volume and long-term blood pressure through stimulation of several tubular transporters of Na+-conversation that are mainly located in the proximal tubule, as well as through its effects in enhancing aldosterone secretion and Na+ reabsorption in the distal tubule. As a potent direct smooth-muscle vasoconstrictor and as an enhancer of ADH and sympathetic nervous system activity, angiotensin II also participates in short-term blood-pressure regulation in disorders associated with volume depletion or circulatory depression. Research has uncovered multiple nonhemodynamic functions that are primarily mediated by binding to T1 receptors of angiotensin II, which are particularly important in the pathophysiology of progressive renal injury (Hall et al., 1999).
Box 5-1 Effects of Angiotensin II Mediated via AT1 and AT2 Receptor Stimulation
Modified from Burnier M, Brunner HR: Angiotensin II receptor antagonists, Lancet 355:637, 2000.
A rise in plasma aldosterone concentration stimulates urinary K+ secretion, thus allowing maintenance of K+ balance. Aldosterone also increases the excretion of ammonium (NH4+) and magnesium (Mg2+) and increases the absorption of Na+ in the distal tubule, both by increasing the permeability of the apical membrane and by increasing the activity of Na+, K+-adenosine triphosphatase (ATPase) (Marver and Kokko, 1983). The net effect is to generate more negative potential in the lumen, a driving force for increased K+ secretion. In addition, aldosterone enhances reabsorption of sodium in the cortical collecting duct through activation of the epithelial sodium-specific channel, ENaC (Greger, 2000). In performing these functions, aldosterone plays a key role in regulating fluid and electrolyte balance. Long-term aldosterone administration to healthy volunteers increases the ECF volume. Clinical edema does not occur, however, because after several days the kidneys “escape” from the Na+-retaining effect while maintaining the K+-secretory effect (August et al., 1958).
The Kidneys and Atrial Natriuretic Peptide
ANP is secreted by atrial monocytes in response to local stretching of the atrial wall in cases of hypervolemia (e.g., congestive heart failure or renal failure) and ultimately results in the reduction of intravascular volume and systemic blood pressure (Brenner et al., 1990). In the kidneys, ANP acts in the medullary collecting duct to inhibit sodium reabsorption during ECF expansion. ANP induces hyperfiltration, natriuresis, and suppression of renin release, and it inhibits receptor-mediated aldosterone biosynthesis (Greger, 2000). In the cardiovascular system, it diminishes cardiac output and stroke volume and reduces peripheral vascular resistance. Some of these effects are mediated through the influence of ANP on vagal and sympathetic nerve activity.
Body Fluid Compartments
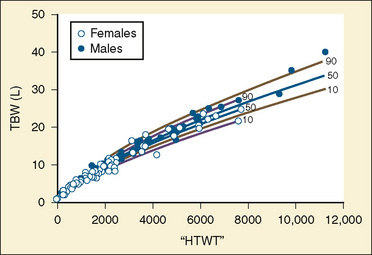
The internal environment of the body consists of fluids contained within compartments. Water accounts for 50% to 80% of the human body by weight. The variation in water content depends on tissue type: adipose tissue contains only 10% water, whereas muscle contains 75% water. Total body water (TBW) decreases with age, mainly as a result of loss of water in ECF. For clinical purposes, TBW is estimated at 60% of body weight in infants older than age 6 months, as well as in children and adolescents. This value is very inaccurate for low–birth-weight premature infants in whom TBW comprises as much as 80% of total body weight (Friis-Hensen, 1971; Kagan et al., 1972). In term infants younger than 6 months of age, TBW may be approximated as 75% of total body weight (Hill, 1990). Newer formulas that consider the height (cm) and weight (kg), but not the degree of adiposity or the child’s surface area, have improved the estimation of TBW, particularly in healthy children between 3 months and 13 years of age (Fig. 5-4) (Mellits and Cheek, 1970; Morgenstern, 2002). TBW can be determined as follows:
Maturation of renal function
Although all nephrons of the mature kidneys are formed by 36 weeks’ gestation during healthy intrauterine life, hyperplasia continues until the sixth postnatal month; thereafter, cell hypertrophy is responsible for increases in renal size. Growth in the size of the kidney tends to be directly proportional to increase in height (Schultz et al., 1962).
While the fetal kidney receives 3% to 7% of cardiac output, RBF increases gradually after birth (Rudolph et al., 1971). RBF, as measured by paraaminohippuric acid (PAH) clearance (CPAH), correlates with gestational age. For example, CPAH is 10 mL/min per square meter at 28 weeks of gestation and 35 mL/min per square meter at 35 weeks of gestation (Fawer et al., 1979). CPAH corrected for body surface area doubles by 2 weeks of age and reaches adult levels at 2 years. Furthermore, changes in RBF are associated with considerable increases in the relative RBF to the outer cortex, where most glomeruli are located (Olbing et al., 1973).
Selected renal functions measured at different ages are summarized in Table 5-3. The GFR in the full-term newborn infant averages 40.6 ± 14.8 mL/min per 1.73 m2 and increases to 65.8 ± 24.8 mL/min per 1.73 m2 by the end of the second postnatal week (Schwartz et al., 1987). GFR reaches adult levels after 2 years of age. Premature newborns have a lower GFR that increases more slowly than that in full-term infants. The low GFR at birth is attributed to the low systemic arterial blood pressure, high renal-vascular resistance, and low ultrafiltration pressure, together with decreased capillary surface area for filtration.
Despite a low GFR, full-term infants are able to conserve Na+ (Spitzer, 1982). This is explained by the existence of glomerulotubular balance, such that as GFR and the filtered load of Na+ increase, so does the ability of the proximal tubule to reabsorb Na+. In contrast, preterm infants have a prolonged glomerulotubular imbalance, so that GFR is high relative to tubular capacity to reabsorb Na+. The glomerulotubular imbalance is caused by structural immaturity of the proximal convoluted tubule and the incomplete development of the transport system responsible for conserving Na+. This, together with poor response of the distal tubule to mineralocorticoids in preterm infants, results in Na+ wastage and susceptibility to hyponatremia.
The tubular mechanisms involved in the excretion of organic acids are poorly developed in neonates. The tubular transport of PAH, which is a weak acid, is around 16 ± 5 mg/min per 1.73 m2 in full-term infants and about half this value in premature babies. It increases with age and reaches adult rates, ranging from 55 to 104 mg/min per 1.73 m2 by 12 to 18 months (Spitzer, 1978). PAH excretion is limited by a number of factors, including low GFR, immaturity of the systems providing energy for transport, and a low number of transporter molecules. This is further accentuated by a low extraction ratio for PAH and other organic acids caused by the predominance of juxtamedullary circulation in the immature kidney, a phenomenon that allows increased shunting of blood through the vasa recta and exclusion of postglomerular blood from the proximal tubular excretory surface (Calcagno and Rubin, 1963).
The kidneys’ ability to concentrate urine is lower at birth, especially in premature infants. After water deprivation in the full-term newborn, urine concentrates to only 600 to 700 mOsm/kg, or 50% to 60% of maximum adult levels. Healthy children ranging from 6 months to 3 years of age who were given 20 mcg of desmopressin intranasally demonstrated a gradual rise in urinary concentration, starting from a mean value of 525 mOsmol/kg to reach a mean maximum plateau of 825 mOsm/kg (Marild et al., 1992). The major cause for the reduced concentration of urine in the neonate is the hypotonicity of the renal medulla (Aperia and Zetterstrom, 1982). Several mechanisms that contribute to interstitial hypertonicity are not well developed, including urea accumulation in the medulla, length of the loop of Henle and the collecting ducts within the medulla, and Na+ reabsorption in the ascending, water-impermeable loop (Trimble, 1970; Horster, 1978; Edwards, 1981). In addition, the collecting duct cells in immature kidneys may be less sensitive to ADH than those of mature nephrons (Schlondorff et al., 1978).
A water-loaded infant can excrete diluted urine with osmolality as low as 50 mOsm/kg. In the first 24 hours of life, however, the infant may be unable to increase water excretion to approximate water intake (Aperia and Zetterstrom, 1982). The diluting capacity becomes mature by 3 to 5 weeks of postnatal life.
Fluid and electrolyte needs in healthy infants and children
The normal need for fluids varies markedly in low–birth-weight and full-term neonates, as well as during infancy and later childhood. This variability in fluid needs is caused by differences in the rate of caloric expenditure and growth, the ratio of evaporative surface area to body weight, the degree of renal functional maturation and reserve, and the amount of TBW at different ages. For instance, compared with older children and adults, infants have greater fluid needs because of higher rates of metabolism and growth; a surface area-to-weight ratio that is about three times greater, resulting in higher insensible fluid loss; and greater urinary excretion of solutes combined with lower tubular concentrating ability, which increases obligatory fluid loss. On the other hand, as previously noted, low–birth-weight and full-term neonates have a greater percentage of TBW compared with older children and adults (Friis-Hensen, 1971; Kagan et al., 1972). This increase in TBW results mainly from expansion of the ECF compartment, which at birth may comprise as much as 50% of the TBW. During the first 3 postnatal days, when this “extra fluid” is eliminated by the kidneys, full-term neonates require less fluid intake (Silverman, 1961; Oh, 1980; Winters, 1982).
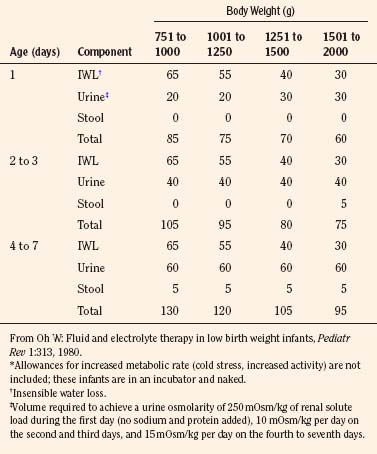
The needs of low–birth-weight infants are more variable and may be markedly altered by relatively minor changes in ambient temperature or by phototherapy (Table 5-4) (Fanaroff et al., 1972; Oh and Karecki, 1972; Wu and Hodgman, 1974). In contrast to more mature infants, the immature skin in very low–birth-weight infants (<1500 g) allows disproportionate evaporative heat loss relative to basal metabolic rate (Levine et al., 1929; Levinson et al., 1966). This greater evaporative heat loss, together with a large body surface area, accounts for the much greater insensible fluid needs in infants with very low birth weight.
TABLE 5-4 Average Fluid Needs of Low–Birth-Weight Infants (mL/kg per 24 hr) During First Week of Life*
Parenteral and Oral Fluids and Electrolytes
Except for the first 3 postnatal days when full-term neonates require only 40 to 60 mL/kg fluid per day, in general, 100 mL of water is needed for each 100 kcal expended. Notably, an additional 15 mL of water is generated endogenously for each 100 kcal used (water of oxidation), which is also available for body functions. In preterm infants, fluid intake may be gradually increased to 150 mL/kg per day, whereas 100 to 125 mL/kg per day generally suffices for infants weighing less than 10 kg. The fluid requirement decreases to 50 mL/kg per day for those weighing 11 to 20 kg and to 20 mL/kg per day for those with body weights above 20 kg. These fluid volumes are sufficient to allow excretion of dietary solute load, as well as to replace insensible fluid loss through the skin, lungs, and intestines (Table 5-5) (Winters, 1982). It should be noted that energy expenditure and, therefore, fluid intake may be significantly increased with stress (Table 5-6) (Holliday et al., 1994).

Full access? Get Clinical Tree