CHAPTER 67
Prostatic Hyperplasia and Male Genital Cancers
Renee McLeod-Sordjan, DNP, FNP-BC, Acute Care-BC
The clinical recognition and evaluation of male genitourinary cancer requiring referral from primary care to urology is challenging. Patients with prostate, testicular, and penile cancer tend to delay seeking clinical attention for more than a year from onset (Skeppner, Andersson, Johansson, & Windahl, 2012). This delay in part can be attributed to embarrassment, refusal of digital rectal examination, and fear of sexual dysfunction. Moreover, delays may also be attributed to clinician’s inability to promote patient adherence to primary care detection of prostate and human papillomavirus (HPV)–related illness in men.
In 2014, an estimated 11,000 American men will be diagnosed with testicular or penile cancer with 700 succumbing to their illness (American Cancer Society [ACS], 2014). An additional estimated 233,000 cases of prostate cancer will also be diagnosed with 29,500 related deaths (ACS, 2014). Male genital cancers are not uncommon, and because of their location, they may be detected at an early stage. Prostate cancer is now the most commonly diagnosed non-cutaneous tumor and the second leading cause of cancer death in men (ACS, 2014).
The primary care clinician also plays a key role in the management of urologic disease as male patients commonly present to them first with concerns, symptoms, or anxieties. Despite referral to a specialist, the primary care provider retains an important responsibility to counsel patients and verify that they are receiving accurate advice. In caring for men throughout their life cycle, the primary care provider offers them support in coping with their symptoms, the morbidities of interventions (e.g., erectile dysfunction and incontinence), and the realities of a much-feared terminal illness. Although the prostate, testes, and penis are in close physical proximity, pathogenesis and management of their disease processes differ so greatly that they will be discussed separately. HPV vaccination will be discussed as a promising primary prevention of penile and prostate cancer.
 PROSTATIC DISEASE
PROSTATIC DISEASE
Symptoms of prostatic disease are frequently characterized by dysuria, hesitancy, frequency, nocturia, urgency, and weak urinary stream, and post-micturition dribbling. In primary care, distinguishing between the differential diagnoses of prostatic disease involves distinguishing between infectious etiology, hyperplasia, and carcinoma. Primary care screening with prostate-specific antigen (PSA) revolutionized clinical diagnostic management of benign prostatic hypertrophy and prostate cancer. Today, the use of PSA dramatically influences detection, diagnosis, and treatment of benign prostatic hypertrophy and its relationship to prostatecancer.
The field of prostate disease is mired in controversy. It is unclear whether early detection via screening for prostate cancer is beneficial and the use of PSA in managing benign prostatic hypertrophy has been clinically updated on numerous occasions. Thus, primary care clinicians have an important responsibility to counsel patients with the best available evidence for their prostatic health. Decisions regarding screening and management should be highly individualized for men to make informed decisions regarding the potential benefits of prostatic disease management. Advances in genetic testing of BRCA in prostate disease may inform future screening guidelines. Technology may facilitate clinicians in diagnosis, but alleviating the psychosocial and cultural concerns of patients requires an empathetic clinician. In caring for men throughout their life cycle, the primary care provider offers them support in coping with their symptoms, the morbidities of interventions (e.g., erectile dysfunction and incontinence), and the realities of a much-feared potentially terminal illness.
Anatomy, Physiology, and Pathophysiology
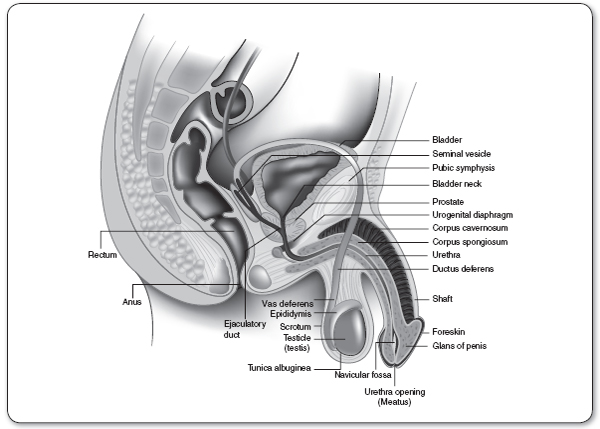
The base of the prostate (Figure 67.1) lies superiorly against the bladder; the apex lies below, against the urogenital diaphragm. The anterior aspect of the prostate lies against the symphysis pubis, whereas the posterior aspect sits in front of the rectum. Only the posterior aspect of the prostate is palpable.
The prostate is composed of both glandular and muscular tissue. The prostate contains multiple epithelial glands that produce a thin, milky secretion. This secretion drains via approximately 25 ducts into the back of the urethra. Smooth muscle in both the prostatic capsule and stroma contracts during ejaculation, expelling the secretion into the ejaculate. The prostatic secretion is alkaline, in contrast to the other components of the ejaculate and to the vaginal pH, both of which are acidic. Because sperm is optimally motile at a higher (i.e., more alkaline) pH, the prostatic secretion may play a role in male fertility by maintaining a suitable pH for sperm.
The prostate gland is divided into three general zones: peripheral, central, and transition.
Approximately 70% of the prostate gland is contained within the peripheral zone, and the majority of prostate cancers originate within this zone. The central zone is 25% of the normal prostate and the site of 5% of prostate cancers. The stroma of the prostate gland is the densest in the central zone. The transition zone is the only site affected by benign prostatic hyperplasia (BPH) and comprises 5% of the prostate. Approximately 10% of prostate cancers originate within the transition zone.
Benign Prostatic Hypertrophy
BPH is not believed to be a risk factor for prostate cancer. BPH occurs primarily in the transitional zone of the prostate, while most prostate cancer originates primarily in the peripheral part of the prostate. The presence of BPH in older men increases the likelihood of a patient being tested for prostate cancer. However, the Prostate Cancer Prevention Trial did not find an association between BPH and an increased risk of prostate cancer (Schenk et al., 2011). BPH is an umbrella term whose symptoms are described in the literature by four different abbreviations that include:
There is poor correlation between urologic symptoms and the presence of prostatic enlargement on rectal examination or by transrectal ultrasonography.
 Lower urinary tract symptoms (LUTS)
Lower urinary tract symptoms (LUTS)
 Benign prostatic enlargement (BPE)
Benign prostatic enlargement (BPE)
 Benign prostatic obstruction (BPO)
Benign prostatic obstruction (BPO)
 Bladder outlet obstruction (BOO)
Bladder outlet obstruction (BOO)
Epidemiolgy
The prevalence of BPH increases with age. Histologically diagnosed BPH increases from 8% in the third decade of life, to 40% to 50% in the fifth through sixth decade of life, to over 80% in octogenarian men. The pathogenesis of BPH is multifactorial and incompletely understood. Over the past decade, the natural progression of BPH has been researched for better understanding. Symptoms of BPH appear slowly and progress over years. The prevalence of moderate to severe LUTS and decreased peak urinary flow rates increase with age. Older age and the presence of androgen in the form of dihydrotestosterone (DHT) is necessary for the development of BPH. Men younger than 40 years with hypogonadism have a decreased risk of BPH development. Increased risk factors associated with BPH include family history of BPH or prostate cancer, alcohol use, diabetes mellitus, inflammation, tumor necrosis factor receptor II, and elevated interleukin-6 concentrations (Parsons et al., 2006; Schenk et al., 2010).
Race and ethnicity appear to be factors in the necessity of surgical intervention for prostatic hyperplasia. Yet, risks of BPH development appear to be more associated with the social determinants of health such as income and access to healthcare rather than race. In a prospective study of 2,488 white men and 4,188 biack men, the prevalence of BPH was significantly associated (p <.05) with a lower income, lower educational status, marital status, and source of insurance (Fowke, Munro, Signorello, Blot, & Penson, 2011). Research has demonstrated that Black men are more likely to require surgery due to a higher symptom burden and stage at initial clinical presentation while Asian counterparts are less likely to be diagnosed or have invasive procedures (Chornokur, Dalton, Borysova, & Kumar, 2011; Kristal et al., 2007). For example, among a study of 45,410 male dentists, veterinarians, pharmacists, optometrists, osteopathic physicians, and podiatrists, Asian men (n = 1,589) were less likely (relative risk: 0.4, 95% CI: 0.20.8) to undergo surgery for BPH as compared with White men (Platz, Kawachi, Rimm, Willett, & Giovannucci, 2000). Recent research suggests that this racial disparity in access to treatment is currently improving with regard to definitive prostatectomy by minimally invasive surgical methods (Trinh et al., 2012).
History and Physical Examination
It is important to conduct a thorough history and physical examination on any man who presents in primary care with LUTS that include increased frequency of urination, noc-turia, hesitancy, urgency, and weak urinary stream. LUTS is defined as including storage and/or voiding disturbances common in aging men due to structural or functional abnormalities in the lower urinary tract or abnormalities of the peripheral and/or central nervous systems that provide neural control of the LUT (McVary et al., 2011). Therefore, LUTS can be due to BPH or systemic diseases in the respiratory, cardiovascular, or renal systems. All men with LUTS should have a digital rectal reexamination (DRE) to assess prostate size, consistency, and to detect signs of malignancy (i.e., nodules, induration, and asymmetry).
The normal consistency of the prostate gland is that of the thenar eminence when contracted—for example, when the thumb is opposed to the little finger. When the muscle is relaxed, it gives the boggy feel of a benign enlarged prostate. Hard nodules are like the bony prominences of the hand; indurated areas are like those of costochondral cartilage. In general, inflammatory nodules are raised; cancerous ones are not. A prostate cancer may be often obvious as a hard mass if detected on DRE. This mass may extend beyond the capsule of the prostate and fix the organ to the pelvic wall.
In patients with prostatic obstruction, it is important to evaluate for possible urinary retention, either acute or chronic. Patients with chronic retention can have massively enlarged bladders and still be relatively or entirely asymptomatic. Approximately 400 mL of urine must be present in the bladder before it can be palpated suprapubically. A more sensitive method is percussion. The presence of a dull percussive note one-finger breadth above the symphysis is a reliable sign that there is at least 100 mL of urine in the bladder (Boyarsky & Goldenberg, 1962).
Clinical Pearls
 Before beginning the examination, inform the patient that it is normal to feel a desire to urinate as the prostate is palpated.
Before beginning the examination, inform the patient that it is normal to feel a desire to urinate as the prostate is palpated.
 The median sulcus is easily palpated, as are two lateral lobes. The seminal vesicles lie superior to the prostate and are not normally palpable.
The median sulcus is easily palpated, as are two lateral lobes. The seminal vesicles lie superior to the prostate and are not normally palpable.
 The examiner can palpate only the posterior surface of the prostate; any anterior lesion will escape detection. This is an important limitation of the DRE.
The examiner can palpate only the posterior surface of the prostate; any anterior lesion will escape detection. This is an important limitation of the DRE.
The size of the prostate on palpation does not correlate with the degree of urethral obstruction. Estimation of prostate size is not particularly useful diagnostically. However, prostate size may influence the choice of therapy for BPH.
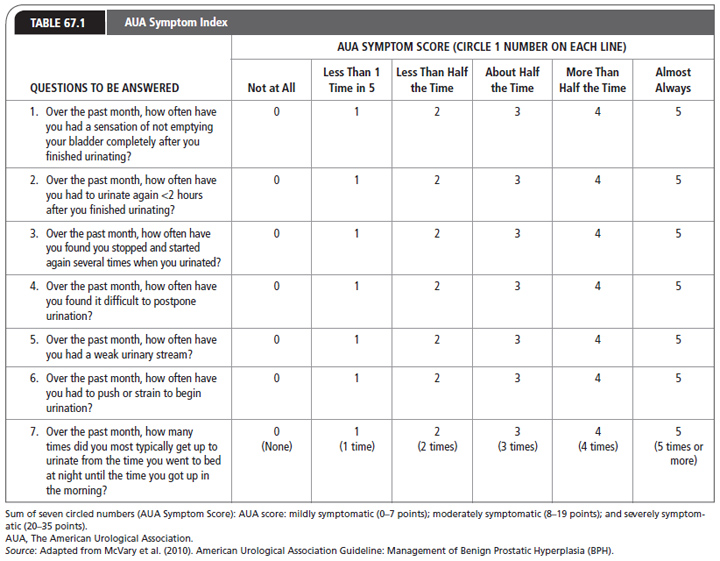
In 2010, The American Urological Association (AUA) updated the screening and evaluation guideline of the Agency for Health Care Policy and Research to evaluate men with BOO. A seven-question symptom index was developed by the AUA to evaluate the response to therapies for BPH (Table 67.1). The index is used in some settings for making clinical decisions (McVary et al., 2011). The AUA symptom index is a patient self-report of symptoms of LUTS. The AUA symptom index is not statistically related to anatomic and physiologic variables, such as the peak urinary flow rate or the post-void residual, which are thought to be associated with urinary obstruction. This tool when combined with a single quality of life question is known as the International Prostate Symptom Score (IPSS). The quality of life question asks: “If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?” The question is rated with a score of 0 through 6 using the descriptors: “delighted, pleased, mostly satisfied, mixed, mostly dissatisfied, unhappy and terrible.” “Delighted” is scored as 6.
Clinical Pearls
 High symptom scores are not diagnostic of prostatic obstruction but merely an indication of patient symptomatology. Patient’s symptoms should dictate proceeding with therapy regardless of presence of obstruction.
High symptom scores are not diagnostic of prostatic obstruction but merely an indication of patient symptomatology. Patient’s symptoms should dictate proceeding with therapy regardless of presence of obstruction.
Diagnostic Criteria
The AUA recommends a urinalysis and a PSA for all men being evaluated for LUTS. A serum creatinine (SCr) is also important to assess renal function status that may be altered by outlet obstruction or prerenal disease. Other diagnostics that may be useful in evaluation of BPH include maximal urinary flow rate, post-void residual urine volume, and urine cytology. The AUA does not recommend pressure low studies or urethrocystoscopy in the routine evaluation of m en with B PH.
Prostate-Specific Antigen
PSA is a protease that functions to liquefy the ejaculate. It is produced throughout the prostate gland and, for practical purposes, is found nowhere else in the body. As the prostate hypertrophy increases with age, more PSA is produced. PSA levels tend to increase with age, so the normal range increases as men grow older. Levels also increase in prostatitis, prostatic massage, perineal trauma, after ejaculation with sexual activity for 48 to 72 hours, and when the prostate is manipulated surgically or by cystoscopy. Levels may be falsely low and not reflect the true presence of prostatic disease (e.g., cancer) in men with hypogonadism and obesity secondary to low circulating testosterone (Parsons et al., 2006).
Difficulty arises in distinguishing PSA elevations from BPH and those from prostate cancer. This generally occurs with PSA levels in the range of 4 to 10 ng/mL. In men with a normal prostate, active PSA is produced by secretory cells within the prostate and altered by proteolysis into inactive PSA and free PSA. Free PSA circulates unbound in the serum. The percentage of free or unbound PSA is lower in the serum of men with prostate cancer compared with those who have a normal prostate or BPH. Therefore, the ratio of free to total PSA and complexed PSA (cPSA) can be utilized as a means of distinguishing between prostate cancer and BPH as a cause of an elevated PSA. Although there again is no consensus on the best threshold value for free PSA, values above 25% reliably predict the absence of clinically significant prostate cancer while a value below 10% is more commonly associated with prostate cancer (Ito et al., 2003; McVary et al., 2010).
The specificity of the serum PSA assay for prostate cancer is lower in men with obstructive symptoms than in asymptomatic men (McDonald et al., 2014). Therefore, interpretation of PSA levels must be adjusted for age, race, and use of 5-alpha-reductase inhibitors. Inhibitors of 5-alpha-reductase (i.e., finasteride and dutasteride) reduce PSA levels by 50%; therefore, normal PSA reference must be halved to evaluate this population diagnostically. PSA levels that suggest risks for cancer, adjusted for race and age, are as follows:
 40 to 49 years old—0 to 2.0 ng/mL (Blacks); 0 to 2.5 (Whites)
40 to 49 years old—0 to 2.0 ng/mL (Blacks); 0 to 2.5 (Whites)
 50 to 59 years old—0 to 4.0 ng/mL (Blacks); 0 to 3.5 (Whites)
50 to 59 years old—0 to 4.0 ng/mL (Blacks); 0 to 3.5 (Whites)
 60 to 69 years old—0 to 4.5 ng/mL (Blacks); 0 to 3.5 (Whites)
60 to 69 years old—0 to 4.5 ng/mL (Blacks); 0 to 3.5 (Whites)
 70 to 79 years old—0 to 5.5 ng/mL (Blacks); 0 to 3.5 (Whites) (Carter et al., 2013).
70 to 79 years old—0 to 5.5 ng/mL (Blacks); 0 to 3.5 (Whites) (Carter et al., 2013).
![]() CLINICAL WARNING:
CLINICAL WARNING:
PSA levels above 10 ng/mL, are more specific for cancer.
Certain medications have been linked to reduction of PSA levels including NSAIDs, acetaminophen, statins, and thiazides but the clinical relevance is unknown. It is uncertain whether the reduction in PSA reflects an actual reduction in prostate cancer risk or rather an effect on PSA production that can potentially alter the screening characteristics of PSA measurement. Initiation of any of these medications may affect PSA velocity, and so should be taken into account when evaluating PSA changes over time.
 Use of PSA as a screening tool for prostate cancer dramatically changed diagnosis of BPH and prostate cancer. However, in 2011, a study demonstrated that mortality from prostate cancer, in the past 20 years, did not differ significantly between men screened with PSA from and those in a control group (Sandblom et al., 2011). The United States Preventive Services Task Force (USPSTF) reported that 80% of men with elevated serum PSA levels have false-positive results and undergo prostatic biopsy (Moyer, 2012; USPTF, 2012). Moreover, the USPTF suggested that for every 1,000 men screened, one man will avoid prostate cancer death. Thus, the USPTF recommended against PSA screening in healthy men. Yet, the USPSTF stressed that men who want to get a PSA test can do so after a clinician explains the limitations and uncertainties.
Use of PSA as a screening tool for prostate cancer dramatically changed diagnosis of BPH and prostate cancer. However, in 2011, a study demonstrated that mortality from prostate cancer, in the past 20 years, did not differ significantly between men screened with PSA from and those in a control group (Sandblom et al., 2011). The United States Preventive Services Task Force (USPSTF) reported that 80% of men with elevated serum PSA levels have false-positive results and undergo prostatic biopsy (Moyer, 2012; USPTF, 2012). Moreover, the USPTF suggested that for every 1,000 men screened, one man will avoid prostate cancer death. Thus, the USPTF recommended against PSA screening in healthy men. Yet, the USPSTF stressed that men who want to get a PSA test can do so after a clinician explains the limitations and uncertainties.
The USPSTF recommendation is not a mandate and the AUA disagrees (Makarov & Partin, 2006). The AUA based their current recommendations on a systematic review summarizing evidence of 300 studies that addressed four outcomes: prostate cancer incidence/mortality, quality of life, diagnostic accuracy, and harms of testing (Carter et al., 2013). The guideline considered patient values and preferences. The current recommendation suggests no screening of men younger than 40 years, aged 40 through 54 years at average risk, older than 70 years of age, or any man whose life expectancy is <10 to 15 years (Carter et al., 2013). In men aged 40 to 54 years at higher risk of prostate cancer (e.g., positive family history or African American race), decisions to screen should be individualized after deliberate considerations of the risks and benefits. Therefore, routine PSA screening is reserved for men aged 55 to 69 years every 2 years. As compared to annual screening, AUA expects that screening intervals of 2 years preserve the majority of the benefits and reduce overdiagnosis and false positives (Carter et al., 2013). Clinicians screening men aged 70 years or older should attempt to identify those who would benefit from prostate cancer treatment and discontinue screening in older men with a PSA less than 3 ng/mL. Men older than 70 years with a PSA below 3 ng/mL have a significantly lower likelihood of being diagnosed with a lethal prostate cancer during the remaining years of life (Carter et al., 2013; McVary et al., 2010).
Decision making may be difficult for clinicians and patients given the change in best practices in prostate screening proposed by newer guidelines. Higher quality decision making occurs when the latest scientific evidence is balanced by patients’ informed cultural values and beliefs associated with each option. Primary care clinicians can support informed consent by reviewing probabilities of the outcomes of options (benefits/harms) and having patients explicitly clarify their values regarding sexual function. Supporting patient involvement within a patient-centered decision-making approach is associated with reduced provider-patient conflict and increased treatment congruent with their wishes (Hoffman et al., 2010; Stacey et al., 2008; Watson et al., 2006).
Two approaches are helpful in discriminating borderline PSA values (4–10 ng/mL) in BPH versus prostate cancer, but neither has gained complete acceptance. The approaches are:
 PSA density: Determined by dividing the PSA level by the weight of the prostate as determined by ultrasound. This theoretically removes the increase in PSA secondary to hypertrophy. Serum PSA is then divided by prostate volume to give a PSA density, with higher PSA density values (>0.15 ng/mL/cc) suggesting prostate cancer while lower values suggest BPH.
PSA density: Determined by dividing the PSA level by the weight of the prostate as determined by ultrasound. This theoretically removes the increase in PSA secondary to hypertrophy. Serum PSA is then divided by prostate volume to give a PSA density, with higher PSA density values (>0.15 ng/mL/cc) suggesting prostate cancer while lower values suggest BPH.
 PSA velocity: The PSA is plotted over time to assess the rate of change. PSA levels are expected to increase with age, but a change in the velocity of the increase is thought to signal malignant prostatic disease. A PSA velocity cutoff of 0.75 ng/mL per year suggests prostate cancer from those with either BPH or no prostate disease with a specificity of 90% to 100% (Carter et al., 2013).
PSA velocity: The PSA is plotted over time to assess the rate of change. PSA levels are expected to increase with age, but a change in the velocity of the increase is thought to signal malignant prostatic disease. A PSA velocity cutoff of 0.75 ng/mL per year suggests prostate cancer from those with either BPH or no prostate disease with a specificity of 90% to 100% (Carter et al., 2013).
Clinical Pearls
 AUA recommends screening in asymptomatic men for prostate cancer 55 to 69 years of age utilizing a risk-benefit ratio. The risks of harm associated with overdiagnosis and surgical complications should be considered before screening other populations aged 40 to 54 years.
AUA recommends screening in asymptomatic men for prostate cancer 55 to 69 years of age utilizing a risk-benefit ratio. The risks of harm associated with overdiagnosis and surgical complications should be considered before screening other populations aged 40 to 54 years.
Maximal Urinary Flow Rate
Maximal urinary flow rates are determined by urodynamic studies. Urodynamics includes a variety of techniques, ranging from the simple to the sophisticated. Uroflowmetry is a simple test commonly used for evaluating symptoms of urinary obstruction. It is the urologic equivalent of the pulmonary flow-volume curve. The patient urinates into a special measuring device that produces a curve of flow versus time. The curve permits normal outflow to be distinguished from the decreased pattern characteristic of prostatic obstruction and the plateau characteristic of bladder neck obstruction. Unfortunately, there are limitations to uroflowmetry. Decreased flow is not diagnostic as it can be caused by bladder decompensation or a lazy detrusor without prostatic obstruction. Normal flow can occur despite obstruction if the detrusor is hypertrophied. Maximum rates >15 mL/s rule in obstruction whereas rates <10 mL/s rule it out. An important caveat that improves use of flow rates clinically is to ensure that prevoided bladder volume is >250 mL and that the voided volume is 150 mL. Men with BPH have better surgical outcomes when maximal flow rates are <10 mL/s.
POST-VOID RESIDUAL URINE VOLUME
A normal post-void residual volume for men is <12 mL of residual urine. Large post-void volumes are possible indicators of BPH, as well as infection or bladder decompensation.
ULTRASONOGRAPHY
Ultrasound is indicated in men with LUTS with high SCr or signs of infection to rule out bladder masses or nephroli-thiasis. It is also useful in presurgical evaluation of men with BPH when considering medical treatment with a 5-alpha-reductase inhibitor versus surgery. Total prostate volume can also be measured by ultrasonography to assess disease progression.
Clinical Pearls
 The routine measurement of SCr levels is not indicated in the initial evaluation of men with LUTS secondary to BPH. Yet, an elevated creatinine level is associated with higher mortality and complication rate after prostate surgery.
The routine measurement of SCr levels is not indicated in the initial evaluation of men with LUTS secondary to BPH. Yet, an elevated creatinine level is associated with higher mortality and complication rate after prostate surgery.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Urine cytology should be performed on men with a smoking history and obstructive symptoms as bladder cancer risk increases in this population.
Treatment Options, Expected Outcomes, and Comprehensive Management
Primary care referral to urology is indicated after initial evaluation if the digital rectal examination clinically suggests prostate cancer. Other indications for referral include hematuria, abnormal prostate-specific antigen (PSA) levels, recurrent urinary tract infection, palpable bladder mass, urethral stricture, and/or a neurological disease. Patients who do not meet the criteria for urologic referral can be managed in one of several ways. The choice is largely dictated by patient preference. Treatment decisions are reached in a shared decision-making process between the clinician and patient. Table 67.3 reviews the algorithm for treatment of moderate to severe
Watchful waiting refers to active surveillance by the patient and clinician. It is the preferred treatment for men with mild symptoms defined by an AUA score <8. It may also be useful in men with moderate to severe symptoms (AUA score >8) without complications of renal insufficiency, urinary retention, or recurrent infection. Watchful waiting may also be the patient’s treatment choice with high AUA scores as the level of symptom distress that individual patients tolerate is highly variable. During watchful waiting, symptom distress may be reduced with simple measures such as avoiding decongestants or antihistamines, decreasing fluid intake at bedtime, and decreasing caffeine and alcohol intake.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Watchful waiting is not considered appropriate in men with large post-void residuals.
Medical Therapy
There are four approaches to the medical therapy of BPH.
 Alpha-adrenergic blockade relaxes the smooth muscle of the prostate.
Alpha-adrenergic blockade relaxes the smooth muscle of the prostate.
 Antiandrogen therapy deprives the prostate of a growth-enhancing factor.
Antiandrogen therapy deprives the prostate of a growth-enhancing factor.
 Combination therapy combines alpha blockade with an an tiandrogen.
Combination therapy combines alpha blockade with an an tiandrogen.
 Anticholinergic therapy.
Anticholinergic therapy.
SELECTIVE ALPHA-1 BLOCKADE
The smooth muscles of the prostate and urinary sphincter respond to alpha-adrenergic stimulation; this is why nasal decongestants have been known to precipitate urinary retention. Selective alpha-1-adrenergic blockers cause relaxation of this smooth muscle and can relieve the symptoms of obstruction.
First-line treatment of BPH is use of selective alpha-1 blockers. In clinical practice, the five selective alpha-blockers used areterazosin, tamsulosin, alfuzosin, doxazosin, and silodosin. They are all long acting and can be given once daily. Prazosin was the first selective alpha-1-adren-ergic blockers marketed. It is no longer recommended first line but remains a less costly generic alternative. Prazosin is usually given twice a day and requires dosage titration. Terazosin and doxazosin also require dosage titration. Table 67.2 presents one method of initiating terazosin and do xazosin therapy.
This class of medications contains all antihyperten-sive medications and may be the ideal agents in an elderly patient with hypertension. Unfortunately, their hypoten-sive action is also the source of their side effects, including orthostatic hypotension, headache, and dizziness. Because of these side effects, it is often suggested that these agents be taken at night and titrated up from very low dosages. These agents may not help reduce bladder pressure. The second-generation alpha-1-adrenergic blockers (e.g., alfuzosin, doxazosin, and silodosin) are more selective alpha-blockers than prazosin and have less hypotensive effects. Other side effects include nasal congestion and retrograde ejaculation (particularly with silodosin).
Initiation of Terazosin and Doxazosin Therapy |
TERAZOSIN STANDARD (APPROPRIATE FOR MOST PATIENTS) | |
Days 1–3 Days 4–14 Weeks 2–6 Week 7 and thereafter | 1 mg 2 mg 5 mg 10 mg |
TERAZOSIN RAPID | |
Days 1–3 Days 4–14 Weeks 2–3 Week 4 and thereafter | 1 mg 2 mg 5 mg 10 mg |
DOXAZOSIN (IMMEDIATE RELEASE) | |
Days 1–3 Days 4–14 Weeks 2–6 Week 7 and thereafter | 1 mg 2 mg 4 mg 8 mg |
DOXAZOSIN (EXTENDED-RELEASE PREPARATION ONLY) | |
Days 1–21 Week 4 and thereafter | 4 mg 8 mg |
Men who are planning cataract surgery are urged to withhold initiation of alpha-1-adrenergic blockers until after surgery. Alpha-1-adrenergic blockers cause iris dilator smooth muscle inhibition that may result in posterior capsule rupture with vitreous loss and postoperative intraocular pressure increase. This syndrome has been named intraoperative floppy iris syndrome (IFIS; Chang & Campbell, 2005). IFIS occurs substantially with tamsulosin and to a lower extent with older generic alpha-1-adrenergic blockers (e.g., terazosin and doxazosin).
![]() CLINICAL WARNING:
CLINICAL WARNING:
Do not initiate alpha-1-adrenergic blockers in men planning cataract surgery due to the risk of IFIS.
5-Alpha-Reductase Inhibitors
These drugs act by reducing the size of the prostate gland. 5-Alpha-reductase inhibitors (5-ARIs) inhibit 5-AR, the enzyme that produces DHT from testosterone. DHT appears to be the agent responsible for prostatic hyperplasia at the subcellular level. 5-ARIs are indicated to prevent symptom progression of BPH and to reduce the risk of urinary retention after future prostate related surgery. They provide an antiandrogenic effect without inducing chemical castration. There are two drugs marketed for BPH; dutasteride and finasteride.
Finasteride was marketed first. Finasteride inhibits only the 5-AR type II isoenzyme, while dutasteride inhibits both types I and II. This difference in activity reduces serum levels of dihydroxytestosterone (DHT) by approximately 70% with finasteride and 95% with dutasteride. Side effects of this class of drugs includes decreased libido, gynecomastia, erectile dysfunction, and ejaculatory disturbances. Treatment for 6 to 12 months is generally needed before prostate size is sufficiently reduced to improve symptoms.
Combination Therapy
Short-term efficacy was not initially demonstrated in large clinical trials of finasteride with either terazosin or doxazosin (Kirby et al., 2003; Lepor et al., 1996). However, long-term efficacy has been demonstrated in the past decade. In clinical studies, combination therapy proved equal to alpha-blocker therapy in efficacy and safety, but superior to 5-ARI therapy alone (Kaplan et al., 2008). The Medical Therapy of Prostate Symptoms supported the use of combination therapy for long-term treatment as superior to both alpha-1 blockers and 5-ARI therapy (Carter et al., 2013; Kaplan et al., 2008). Combination therapy reduced the risk of clinical progression of BPH by 66%, significantly greater than with either drug alone (Kaplan et al., 2008). Analysis of the number needed to treat to prevent one instance of overall BPH progression was 8.4 for combination therapy, 13.7 for doxazosin, and 15.0 for finasteride (Tacklind, Fink, Macdonald, Rutks, & Wilt, 2010). Adverse effects with combination therapy and monotherapy are similar with the exception of abnormal ejaculation, peripheral edema, and dyspnea, which were more common with combination therapy.
Anticholinergic Agents
Anticholinergic agents block muscarinic bladder receptors M2 and M3. While M2 receptors are mainly within the bladder, the M3 receptors are primarily responsible for bladder contraction. Blocking these muscarinic receptors results in a reduction in smooth muscle tone and theoretically an amelioration of excess muscle contraction. These drugs are therefore helpful in treating overactive bladder symptoms in men and women. In men with BPH and bladder dysfunction, these drugs may be helpful adjuvant therapy. Men with high post-void residuals should not be treated with anticholinergics as they cause urinary retention. Currently tolterodine and solifenacin have demonstrated efficacy in clinical trials when used as an adjuvant with tamsulosin. Although not specifically approved for the use of BPH, research has demonstrated that the combination of tamsulosin and tolterodineor solifenacin significantly improved BPH symptoms compared to placebo and monotherapy with either agent (Carter et al., 2013; Yamaguchi et al., 2011).
Referral Points and Clinical Warnings
Referral to a urologist is appropriate in several situations. Acute retention mandates urgent consultation. Stones, recurrent infections, and persistent or painless hematuria should all be given prompt urologic evaluation. Patients with refractory and troublesome symptoms should also be referred to a urologist.
Surgical Therapy
Table 67.3 details the surgical interventions available for men with BPH. They range from open prostatectomy, transurethral to minimally invasive laser therapy procedures.
Treatment of Moderate to Severe BPH |
 Watchful waiting
Watchful waiting
 Medical therapies
Medical therapies
 Alpha-blockers
Alpha-blockers
– Alfuzosin 10 mg QD (no titration required)
– Doxazosin (see text for titration schedule)
– Tamsulosin 0.4 mg QD (1st 2 weeks) titrate 0.8 mg QD
– Terazosin (see text for titration schedule)
– Silodosin 8 mg QD (no titration required)
 5-Alpha-reductase-inhibitors (5-ARIs)
5-Alpha-reductase-inhibitors (5-ARIs)
– Dutasteride 0.5 mg PO QD
– Finasteride 5 mg PO QD
 Combination therapy
Combination therapy
– Alpha-blocker and 5-alpha-reductase inhibitor
– Alpha-blocker and anticholinergics
 Anticholinergic agents
Anticholinergic agents
– Tolterodine 4 mg PO QD (long-acting formulation)
– Solifenacin 5 mg PO QD
 Surgical therapies
Surgical therapies
 Minimally invasive therapy
Minimally invasive therapy
– Transurethral needle ablation (TUNA)
– Microwave thermotherapy (TUMT/TRMT)
 Invasive therapy
Invasive therapy
 Open prostatectomy
Open prostatectomy
 Transurethral holmium laser ablation of the prostate (HoLAP)
Transurethral holmium laser ablation of the prostate (HoLAP)
 Transurethral holmium laser enucleation of the prostate (HoLEP)
Transurethral holmium laser enucleation of the prostate (HoLEP)
 Holmium laser resection of the prostate (HoLRP)
Holmium laser resection of the prostate (HoLRP)
 Photoselective vaporization of the prostate (PVP)
Photoselective vaporization of the prostate (PVP)
 Transurethral incision of the prostate (TUIP)
Transurethral incision of the prostate (TUIP)
 Transurethral vaporization of the prostate (TUVP)
Transurethral vaporization of the prostate (TUVP)
 Transurethral resection of the prostate (TURP)
Transurethral resection of the prostate (TURP)
BPH, benign prostatic hyperplasia.
Minimally Invasive Surgical Therapy
TRANSURETHRAL RADIOFREQUENCY NEEDLE ABLATION
Transurethral radiofrequency needle ablation (TUNA) of the prostate utilizes a cystoscope-like device that emits radiofrequency energy sufficient to heat the prostate to a temperature exceeding that necessary to cause prostatic tissue necrosis. The concept is to heat the transition zone of the prostate while sparing the urethral mucosa, thus preserving the mucosa, reducing pain, and improving patient tolerance. Over time, the necrotic tissue will be absorbed thereby reducing prostatic volume. TUNA is a good procedure for men with significant comorbid disease with moderate BPH. However, it is less efficacious than TURP and should not be used in patients with urinary retention or compromised renal function caused by obstructive uropathy.
TRANSURETHRALLY MICROWAVE THERMOTHERAPY/TRANSRECTAL MICROWAVE THERMOTHERAPY
Microwave thermotherapy has evolved through several different iterations over the past 15 years. Microwave thermotherapy relieves symptoms of BPH by inducing hypothermia transurethrally (TUMT) or transrectally (TRMT). Microwave techniques vary with the degree of heating from thermotherapy to thermoablation. Thermotherapy refers to heating prostatic tissue to temperatures higher than 45°C (113°F), while thermoablation refers to heating to higher temperatures ranging from 60°C to 75°C (140–167°F). Microwave thermotherapy improves urinary flow rates but retreatment with more invasive surgical intervention is usually necessary.
Invasive Surgical Intervention
Prostatectomy can be accomplished transurethrally, suprapubically, or perineatly. When surgery is chosen for BPH, transurethral resection of the prostate (TURP) is used in most cases.
LASER PROSTATECTOMY
Laser prostactomy has evolved from coagulation to enucleation, ablation, or resection with the holmium laser (holmium laser enucleation of the prostate [HoLEP]; holmium laser ablation of the prostate [HoLAP]; holmium laser resection of the prostate [HoLRP]). Laser prostatectomy is not as efficacious as transurethral resection prostatectomy (TURP) and requires more reoperations. TURPs also required less operating room time but resulted in more blood loss, longer catheterization times, and longer hospital stays. Laser-treated subjects in contrast were less likely to require transfusions (<1% vs. 7%) or develop strictures (4% vs. 8%), and their hospitalizations were 1 to 2 days shorter. Therefore, laser prostatectomy may have a role in subjects with severe anemia or who require bloodless surgical interventions for cultural reasons (i.e., Jehovah Witnesses).
VAPORIZATION OF THE PROSTATE
Vaporization can be performed either photosetectively via laser therapy (PVP) or through transurethral approach (TUVP). TUVP is an adaptation of an older laser vaporization technique. Compared to TURP, TUVP improves urinary low rates and quality of life scores (Carter et al., 2013). However, the rates of postoperative dysuria and urinary retention, as well as the need for catheterization, appear to be higher. TUVP and PVP remain good options for patients who are anticoagulated or who cannot tolerate general anesthesia. Reoperation rates were higher with TUVP an d PVP tha n with TURP.
TRANSURETHRAL RESECTION OF THE PROSTATE
When surgery is chosen for BPH, TURP is used in most cases. It produces immediate and dramatic reductions in obstructive symptoms and is considered the gold standard against which other BPH therapies are assessed.
TURP involves the surgical removal of the prostate’s inner portion via an endoscopic approach through the urethra, with no external skin incision. Historically, this procedure was the most common active treatment for symptomatic BPH but potential morbidities, desire to shorten catheter dwell time and pressure to reduce hospital length of stay have fostered the development of the alternative procedures discussed in this section.
The morbidities of TURP need to be explained carefully to patients. These morbidities in part relect the patient population who undergo the procedure—elderly men who often have comorbid conditions. The risks of TURP include TURP syndrome (dilutional hyponatremia after irrigation), urinary retention, postoperative sepsis, hemorrhage, myocardial infarction, stroke, incontinence, erectile dysfunction, retrograde ejaculation, and the need for retreatment. TURP syndrome is a potentially lethal hyponatremia and may clinically present with nausea, vomiting, abdominal pain, confusion, seizures, and coma.
Surgical safety appears to have improved most of these complications over time. A comparison of surgical outcomes in the period from 2000 to 2005 with those from 1979 to 1994 found lower rates of urinary tract infection (1.7% vs. 8.2%), urinary retention (3% vs. 9%), and TURP syndrome (0% vs. 1.1%; Rassweiler, Teber, Kuntz, & Hofmann, 2006). In addition, postoperative prostatic bleeding can be managed effectively with the use of finasteride.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Prostate cancer is identified incidentally in about 5% of patients undergoing TURP. Transrectal ultrasound-guided biopsy of the prostate should be performed 3 months after the TURP to rule out malignancy in patients at high risk for prostate cancer (e.g., family history, African American, or PSA above 10).
TRANSURETHRAL INCISION OF THE PROSTATE
Transurethral incision of the prostate (TIP) refers to a procedure in which a longitudinal incision is made in the prostate gland widening the bladder neck and prostatic urethra without removal of any prostate tissue. TIP is an alternative to TURP that is particularly appropriate for men with small prostates. It involves making an incision from the bladder neck to the verumontanum. In properly selected patients, it is as effective as TURP and is associated with fewer side effects, including less retrograde ejaculation.
OPEN PROSTACTEOMY
Open prostatectomy is not a mainstay of surgical interventions for BPH and occurs in <5% of cases.
Teaching and Self-Care
The primary care provider and the patient must work together collaboratively to ensure that the patient makes a fully informed decision regarding treatment. Patients should understand that the symptoms of BPH wax and wane with time. There are several simple measures that can be useful to ameliorate them. Patients can keep a log of when and how much they urinate; this is called a frequency-volume log. The data derived from the log can help them to adjust their fluid intake to avoid troublesome nocturia. A bedside urinal can ease the logistical problems associated with nocturia. Kegel exercises and bladder training may also be helpful. If caffeine and alcohol are problems, intake should be minimized or avoided entirely.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Referral to a urologist is appropriate in several situations. Acute retention mandates urgent consultation. Stones, recurrent infections, and persistent hematuria should all be given prompt urologic evaluation. Patients with refractory and troublesome symptoms should also be referred to a urologist.
 PROSTATE CANCER
PROSTATE CANCER
Prostate cancer is the most common type of cancer in men and the second leading cause of male cancer death accounting for 14.4% of all new cancer cases in the United States (ACS, 2014). In 2014, approximately 233,000 American men will be diagnosed with prostate cancer and 29,500 will die because of it (Seigel, Ma, Zou, & Jemal, 2014). There is an estimated 2.6 million American men currently living with prostate cancer (Seigel et al., 2014). Diagnosis of prostate cancer has increased in large part secondary to PSA screening with 93% of prostate cancer cases by stage found localized or with regional spread. Prostate cancer survival is multifactorial and especially determined by the extent of tumor at the time of diagnosis. The 5-year relative survival among men with localized prostate cancer or with regional spread is 99.2% compared with 31.9% among those diagnosed with distant metastases (Carter et al., 2013; Howlader et al., 2013).
Epidemiology
Adenocarcinoma accounts for 95% of malignancies of the prostate. The remaining 5% of prostate cancers include transitional cell carcinoma, carcinosarcoma, basal cell carcinoma, lymphomas, or stromal sarcoma. Transitional carcinoma (a.k.a. urothelial carcinoma) is usually due to direct extension of bladder carcinoma into the prostatic urethra or prostatic ducts. Transitional carcinoma accounts for 1% to 4% of prostate cancer. The remaining histologic types of prostate cancer are exceedingly rare.
Seventy percent of prostate cancer is diagnosed in men older than 65 years. The incidence of prostate cancer in African American men is more than twice the incidence identified in Caucasian men (228.5/100,000 vs. 144.9/100,000). Mortality is also higher in African American men where death rates are 50.9/100,000 versus 21.2/100,000 in their Caucasian counterparts (Howlader et al., 2013). In addition to race, age above 80 years, family history of prostate cancer, smoking, alcohol, vitamin E supplementation, folic acid supplementation, and diets high in dairy foods have been associated with higher risk of prostate cancer (Dennis & Hayers, 2013; Figueiredo et al., 2009; Hickey, Do, & Green, 2013; Klein et al., 2011). Use of finasteride and dutasteride has demonstrated a lower risk of developing adenocarcinoma but it remains unclear whether morbidity and mortality are improved (Andriole et al., 2010).
BRCA Testing
A family history of prostate cancer increases the risk of cancer development. Studies have supported that men with a BRCA2 mutation have between a five- and ninefold increased risk of prostate cancer by age 65 years (Alanee, Glogowski, Schrader, Eastham, & Offit, 2013). The risk of prostate cancer in men with a BRCA1 mutation also possibly appears to be elevated by about threefold (Mitra et al., 2011). Prostate cancer in BRCA1 and BRCA2 carriers, especially those of men with mothers with breast cancer is more aggressive and has poorer survival outcomes relative to the general population (Levy-Lahad & Friedman, 2007; Thorne et al., 2011). Future guidelines may support BRCA testing in men at high risk of prostate cancer. Presently, primary care clinicians need to be aware that positive BRCA testing in women for breast cancer has implications of increased risk of prostate cancer in their sons.
As aforementioned, the USPTF does not recommend screening for prostate cancer with a PSA, because there is considerable evidence screening has limited benefit, or even worse, that the harms of unnecessary biopsies outweigh the benefit. In addition to the PSA having limited benefit for screening, it also cannot differentiate between aggressive and nonaggressive prostate cancers. PSA is useful in predicting future risk of prostate cancer; however, the interpretation of PSA levels should always take into account the presence of infection, age, and use of 5-ARIs. In June 2012, the Food and Drug Administration (FDA) approved the Prostate Health Index (PHI) to improve risk assessment of slow growing prostate cancers and reduce the number of biopsies performed. PHI was developed as a combination of PSA, free PSA, and a PSA precursor form called -2 proPSA to calculate the probability of prostate cancer prior to biopsy (Nichol et al., 2012). Research is now focused on newer markers (e.g., PCA 3) to predict prostatic malignancy prior to biopsy (Ferro et al., 2012; Perdonà et al., 2013). Research is presently inconclusive but these markers remain a hopeful area of scientific exploration to reduce unwarranted prostate biopsy.
Diagnostic Criteria
Prostate cancer is usually asymptomatic and diagnosed as a result of an elevated PSA test. Eighty percent of men currently diagnosed with prostate cancer undergo a biopsy because of a suspicious serum PSA. However, digital rectal examination by a clinician retains an important role for early detection as 20% of cases have a prostate nodule that prompts the biopsy. Biopsy is performed via a transrectal approach usually guided by ultrasound; however, an MRI-targeted biopsy may improve diagnosis. MRI-targeted biopsies have utility in patients with rising PSA levels and several negative biopsies, and those men with prostate cancer who will be managed with active surveillance. In comparison with digital rectal examination and ultrasound, MRI demonstrates higher accuracy for the assessment of bilobar prostatic disease.
Symptoms generally occur in the context of metastasis. Metastatic prostate cancer presents as bony pain or neurologic compromise. Bone scans and PET scans are usually unnecessary to assess metastasis in asymptomatic patients with serum PSA level <20 ng/mL or in the presence of well-differentiated tumors. Local extension can cause either urinary obstruction or erectile dysfunction. Prostate cancer can cause local pain, hematuria, and hematospermia. Hematuria and hematospermia are uncommon presentations of prostate cancer but their presence in older men should prompt the primary care clinician to consider prostate cancer within their differential diagnosis.
![]() CLINICAL WARNING:
CLINICAL WARNING: