and Richard A. Jaffe2
(1)
David Geffen School of Medicine at UCLA, Los Angeles, California, USA
(2)
Stanford University School of Medicine, Stanford, California, USA
Keywords
BlindnessIONPositioningFluid therapy blood pressureAnemiaUnanticipated blindness is a devastating event for both patients and medical personnel when it is discovered following emergence from anesthesia and surgery. It is equally unsettling when it occurs in a colleague [1]. As a complication that is unexpected by all parties, it leads to introspection, analysis of the specific anesthetic and surgical procedure, and attempts to explain why it happened. Usually no clear explanation is forthcoming, and all involved are left with the unsettling question “Was something done wrong to cause this?” The patients and their families who must forever live with the consequences of unexpected blindness are adamant in their desire for a full understanding of why it occurred. When no good explanation for the cause of the blindness is given, these patients and families turn to the legal system for redress and compensation.
Postoperative blindness can occur following many types of surgical procedures, but the most frequent, and therefore problematic for anesthesia providers are those occurring following spine surgeries in the prone position. It may occur in patients who are otherwise “healthy”. It also occurs in patients with known hypertension, diabetes and coronary artery disease without any concomitant adverse effects on other organs such as the heart, brain or kidneys. To understand this problem more fully, the American Society of Anesthesiologists (ASA) appointed a 12-member Task Force to “review and assess the current scientific literature; obtain expert consensus and public opinion; and develop a practice advisory”. The practice advisory was published in 2006, and its specific advisories will be addressed in this chapter [2]. As well, in 1999 the Committee on Professional Liability of the ASA established an ASA Postoperative Visual Loss (POVL) Registry to collect cases of visual loss after non-ocular surgery. Data collection was from two sources: voluntary submission utilizing a previously developed data collection form; and from the ASA Closed Claims Project. In October 2006, Lee et al. published an analysis of 93 spine surgery cases with postoperative visual loss collected by the POVL Registry [3]. All the patients in the report had their operations over a 17 year period between 1987 and 2004. Both the Task Force and the Registry concluded that the cause of blindness in most of the patients investigated was due to ischemic optic neuropathy (ION) of unknown etiology, and developed several recommendations for consideration.
Incidence
The reported incidence of blindness following posterior spine surgery ranges from 0.013 to 0.36 % [4, 5], which suggests that the event is rare. However, this incidence is misleading for a variety of reasons. First, because until recently there was no required national reporting mechanism, not all cases were included in retrospective analyses of blindness after spine surgery. Second, there are many spine operations that are minor in terms of duration, extent of the surgical intervention, and blood loss (i.e.: diskectomy), which skew the denominator in the calculation of incidence, and greatly lower the true incidence for patients undergoing major posterior spine surgery such as spinal fusion with instrumentation. A better analysis of incidence would be to compare the number of cases of blindness seen with the number of cases of major posterior spinal surgery exceeding a specific time period, or with time as a continuous variable. Third, although we do not know the total number of major posterior spine surgeries performed between 1987 and 2004, the fact that the Registry was able to identify 83 cases of ION in that interval suggests that it is an ongoing problem of some magnitude. Finally, events labeled as “rare”, regardless of severity, tend to receive less attention in busy medical practices because of the expectation that it will not happen. A recent study by Rubin et al. suggests that the incidence of ION decreased 2.7 fold from 1998 to 2012 [6]. The authors did not determine the cause(s) of this decrease. They did confirm earlier findings that aging, male sex, transfusion and obesity all increase the risk of ION. However, regardless of incidence, it is essential to do all that we can as anesthesia providers to minimize or prevent this event. To achieve this goal, certain issues need clarification and a plan of action developed that has the consensus of the anesthesia community.
Etiology
Blindness following posterior spine surgery may result from either central retinal artery occlusion (CRAO) or anterior or posterior ischemic optic neuropathy (AION/PION) [7] (Fig. 13.1). Since the clinical manifestations of AION and PION are similar, they are typically considered together as ischemic optic neuropathy (ION). ION is more common than CRAO. In the Registry study, 83 of 93 patients had ION; the remaining ten had CRAO. CRAO is usually unilateral whereas ION is almost always bilateral. It is generally believed that CRAO is caused by prolonged external pressure on the globe, while there is no established etiology for ION. Although blindness can occur at any age, it is most common in patients over age 40. Recovery of meaningful vision virtually never occurs with either injury.
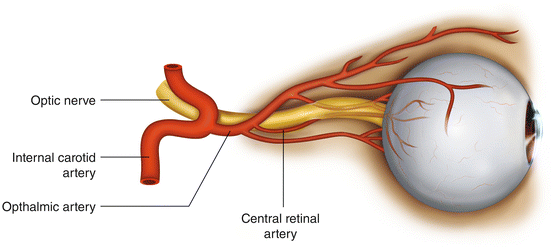

Fig. 13.1
Structural anatomy of the optic nerve and arteries
Prevention of CRAO
Since CRAO is believed to be due to prolonged external pressure on the eyes, prevention is relatively straightforward. Placing the head in Mayfield 3-point fixation will eliminate any such pressure, and is a good choice if a prolonged operation is anticipated. Properly applied, the risks from using this head holder are inconsequential relative to the benefit. Alternatively, there are several available foam or gel-filled head holders with cutouts for the eyes, nose and chin. There is no evidence that one type is better than another, so long as the head is held in a neutral position, and all of the downward pressure is applied on the forehead and cheeks, and not on the eyes, nose or chin. The Task Force could not agree on whether eye checks should be performed by the anesthesia provider and how often. Certainly if technically feasible , it is probably prudent to do so periodically, recognizing that the examination itself may cause undesired head movement resulting in ocular pressure. Head movement for visualization of the eyes can be minimized by using a mirrored head holder (e.g. ProneViewTM) (Fig. 13.2).
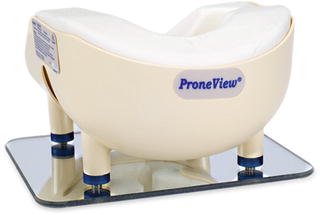

Fig. 13.2
ProneView Protective Helmet System. Courtesy of Dupaco, Inc
Prevention of ION
Since the etiology of ION is unknown, it is impossible to provide any scientifically based recommendations for what to do to prevent it. The Task Force was not able to identify any predisposing medical conditions or any preoperative tests that might indicate susceptibility to ION. However, it did offer eight advisories for the management of “high risk” patients undergoing posterior spine surgery.
Task Force Advisories for “High Risk” patients |
• Avoid direct pressure on the eyes |
• Keep head in neutral position at or above heart level |
• Consider insertion of arterial and central venous catheters |
• Use colloid and crystalloid fluids |
• Check hematocrit periodically |
• Consider staging the operations |
• Consider advising the patient of the possibility of blindness |
• Evaluate the patient early postoperatively for blindness

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



