Fig. 4.1
Sagittal T2-weighted MRI of an epidural abscess (arrows, arrowheads) from an SCS trial
Systemic infections should be treated and under good control prior to moving forward. If any evidence of potential bacteremia exists, the benefit of the stimulation system should be carefully weighed prior to moving forward. In the case of local infections such as cellulitis, the case should be delayed until proper evaluation and treatment can be arranged. This danger should be considered when the patient has had a recent infection in the area of needle insertion. This is not an uncommon concern when considering SCS as part of an algorithmic process for the treatment of intractable disorders. It is for these reasons, the authors advocate getting a basic complete blood count (CBC) prior to implant. Urinalysis may also be helpful in those patients with a history of urinary tract diseases or risks.
4.3.2 Coagulopathy
Bleeding is a concern in any surgical procedure, but probably none more so than in any case in which epidural access is involved. An epidural hematoma is a tragic and catastrophic complication. In a healthy, uncomplicated patient, the incidence is as low as 1 in 40,000. Given the plethora of data as they pertain to epidural hematomas, there is a predictability of sorts as to when the risk may be higher at certain points than others (Fig. 4.2). As a result, guidelines are now in place that give some safeguards to lower the risk.
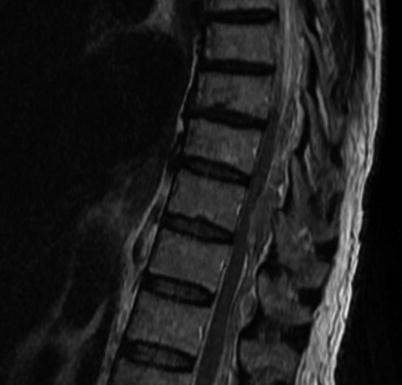

Fig. 4.2
Sagittal T2-weighted MRI of an epidural hematoma following spinal cord stimulator implant
The patient should have no untreated bleeding disorders. Prior to implanting the device the patient should be questioned concerning diseases that affect clotting, liver function, and platelet activity. A preoperative workup would include a CBC including a platelet count.
International Normalized Ratio (INR)—the most predictive of potential complication
Prothrombin time/partial thromboplastin time (PT/PTT) and bleeding times—not as reliable, but may be helpful as general sources of information.
Platelet function assay studies—a new test area that may lend information for patients on drugs that affect platelet function.
Special attention should be paid to assess whether the patient is taking any medication that may put she or he at risk for increased bleeding. The guidelines of the American Society of Regional Anesthesia (ASRA) on bleeding and medication should be reviewed when doing a patient evaluation (Table 4.1). If it is discovered the patient is taking a medication listed on the ASRA guidelines that may affect bleeding, the prescribing physician should be consulted to determine if he or she can safely discontinue those medications for the appropriate length of time prior to invading the epidural space. At this point, this is the best available guidance, but it was not designed for neuromodulation devices. It would be preferable in the future to have guidance directly applicable to this field.
Table 4.1
American Society for regional anesthesia consensus guidelines on anticoagulation and neuraxial blocks 2010
Antiplatelet medications | |
Nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 (COX-2) inhibitors | May continue |
Aspirin (low dose) | Preferably stopped 2–3 d prior for thoracic and cervical epidurals, may be continued in lumbar epidurals. |
Thienopyridine derivatives | |
Clopidogrel (Plavix) | Discontinue for 7 d |
Ticlopidine (Ticlid) | Discontinue for 14 d |
Prasugrel | Discontinue for 7–10 d |
Glycoprotein IIb/IIIa inhibitors | |
Abciximab (ReoPro) | 24–48 h |
Eptifibatide (Integrilin) | 8 h |
Tirofiban (Aggrastat) | 8 h |
The above are the times suggested until normal platelet aggregation is regained. Neuraxial techniques should be avoided until platelet function is recovered | |
Warfarin (coumadin) | |
Check INR (coagulation response time) INR should be normalized | |
2003 Guidelines stated INR <1.5 | |
Heparin | |
Subcutaneous heparin | No contraindication (dosing twice daily and <10,000 U total daily dose) |
Block should be performed before the injection is given | |
If frequency is 3 times a day or >10,000 U, safety has not been established | |
Intravenous heparin | Wait 2–4 h after last dose of IV heparin |
Wait a minimum of 1 h after neuraxial block to restart IV heparin | |
Low-molecular-weight heparin (LMWH) | |
LMWH preoperative—wait time from last dose | |
Enoxaparin (Lovenox) 0.5 mg/kg BID (prophylactic dose) | 12 h |
Enoxaparin (Lovenox) 1 mg/kg BID (prophylactic dose) | 24 h |
Enoxaparin (Lovenox) 1.5 mg/kg QD | 24 h |
Dalteparin (Fragmin) 120 U/kg BID | 24 h |
Dalteparin (Fragmin) 200 U/kg QD | 24 h |
Tinzaparin (Innohep) 175 units/kg QD | 24 h |
LMWH postoperative—time to wait after procedure before restarting | |
Twice-daily dosing | 24 h |
Once-daily dosing | 6–8 h |
Patients with epidural catheter on LMWH | |
Enoxaparin (Lovenox) 0.5 mg/kg: remove the catheter ≥12 h after last dose | |
Enoxaparin (Lovenox) 1–1.5 mg/kg, dalteparin (Fragmin), or tinzaparin (Innhop): remove the catheter ≥24 h after last dose | |
The catheter should be removed as soon as possible | |
Restart the LMWH ≥2 h after catheter removal | |
Specific Xa inhibitor: fondaparinux (arixtran) | |
No definitive recommendations | |
If neuraxial performed: recommend single-needle atraumatic placement or alternate thromboprophylaxis, avoid indwelling catheter | |
Fibrinolytic/thrombolytic drugs | |
Absolute contraindications | |
Thrombin inhibitors | |
No definitive recommendations

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |




