Pneumonia in the Ventilator-Dependent Patient: Introduction
Ventilator-associated pneumonia (VAP) is the most frequent intensive care unit (ICU)-acquired infection among patients receiving mechanical ventilation.1 In contrast to infections of other frequently involved organs (e.g., urinary tract and skin), for which mortality is low, ranging from 1% to 4%, the mortality rate for VAP, defined as pneumonia occurring more than 48 hours after endotracheal intubation and initiation of mechanical ventilation, ranges from 20% to 50% and can even be higher in some specific settings or when lung infection is caused by high-risk pathogens.1–3 Although the attributable mortality rate for VAP is still debated, it has been shown that these infections prolong both the duration of ventilation and the duration of ICU stay.1,2 Approximately 50% of all antibiotics prescribed in an ICU are administered for respiratory tract infections.4 Because several studies have shown that appropriate antimicrobial treatment of patients with VAP significantly improves outcome, more rapid identification of infected patients and accurate selection of antimicrobial agents represent important clinical goals.2 Consensus, however, on appropriate diagnostic, therapeutic, and preventive strategies for VAP has yet to be reached. In this chapter, we summarize published studies on epidemiology, diagnosis, treatment, and prevention of nosocomial pulmonary infection in critically ill patients mechanically ventilated in the ICU, and present our experience with this infection.
Epidemiology
Accurate data on the epidemiology of VAP are limited by the lack of standardized criteria for its diagnosis. Conceptually, VAP is defined as an inflammation of the lung parenchyma caused by infectious agents not present or incubating at the time mechanical ventilation was started. Despite the clarity of this conception, the past three decades have witnessed the appearance of numerous operational definitions, none of which is universally accepted. Even definitions based on histopathologic findings at autopsy may fail to find consensus or provide certainty. Pneumonia in focal areas of a lobe may be missed, microbiologic studies may be negative despite of presence of inflammation in the lung, and pathologists may disagree on the findings.5 The absence of a “reference standard” continues to fuel controversy about the adequacy and relevance of many studies in this field.
The exact incidence varies widely depending on the case definition of pneumonia and the population being evaluated.6–10 All studies, however, have confirmed that nosocomial pneumonia is considerably more frequent in ventilated patients than in other ICU patients, with an incidence increasing by as much as sixfold to 20-fold in this subset of patients.11,12 VAP occurs in 9% to 27% of all intubated patients and its incidence increases with duration of ventilation.10,13 The risk of VAP is highest early in the course of hospital stay and is estimated to be 3% per day during the first 5 days of ventilation, 2% per day during days 5 to 10 of ventilation, and 1% per day beginning from day 11.13 Because most mechanical ventilation is short term, approximately half of all episodes of VAP occur within the first 4 days of mechanical ventilation. In a large epidemiologic study, independent predictors of VAP retained by multivariable analysis were a primary admitting diagnosis of burns, trauma, central nervous system disease, respiratory disease, cardiac disease, mechanical ventilation during the preceding 24 hours, witnessed aspiration, and use of paralytic agents. Exposure to antibiotics conferred protection, but this effect was attenuated over time.13 According to four studies, the VAP rate was higher in patients with acute respiratory distress syndrome (ARDS) than other ventilated patients, affecting between 34% and more than 70% of patients with ARDS and often leading to the development of sepsis, multiorgan failure, and death.14–17
Mechanically ventilated patients in the ICU with VAP appear to have a twofold to 10-fold higher risk of death as compared with patients without pneumonia. Although these statistics indicate that VAP can be lethal, previous studies have not demonstrated clearly that pneumonia is responsible for the higher mortality rate of these patients.18 It is often difficult to determine whether ICU patients with severe underlying illness would have survived if VAP had not occurred. VAP, however, has been recognized in several case-controlled studies or studies using multivariate analysis as an important prognostic factor for different groups of critically ill patients.18–23
Other factors beyond the simple development of VAP, such as the severity of the disease, the responsible pathogens, or the appropriateness of initial treatment, may be more important determinants of outcome for patients in whom pneumonia develops.24 Indeed, it may be that VAP increases mortality only in the subset of patients with intermediate severity of illness,23 when initial treatment is inappropriate,25–32 and/or in patients with VAP caused by high-risk pathogens, such as Pseudomonas aeruginosa.24,33 Patients with very low severity and early onset pneumonia caused by organisms such as Haemophilus influenzae or Streptococcus pneumoniae have excellent prognoses with or without VAP, whereas very ill patients with late-onset VAP, occurring while they are in a quasi-terminal state, would be unlikely to survive. Using a multistate progressive disability model that appropriately handled VAP as a time-dependent event in a high-quality database of 2873 mechanically ventilated patients, Nguile-Makao et al showed that VAP attributable mortality was 8.1% overall, varying widely with case mix, severity at admission, time to VAP onset, and severity of organ dysfunction at VAP onset.24 These results are consistent with the 10.6% value obtained in five German ICUs using also a multistate model progressive disability model.34
It is impossible to evaluate precisely the morbidity and excess costs associated with VAP. All studies, however, have shown clearly that patients with VAP have prolonged duration of mechanical ventilation and lengthened ICU and hospital stay as compared with patients who do not have VAP.1,2,35,36 Summarizing available data, VAP appears to extend the ICU stay by at least 4 to 6 days, with the attributable ICU length of stay being longer for medical than surgical patients and for patients infected with “high-risk” as opposed to “low-risk” organisms.37 The prolonged hospitalization of patients with VAP underscores the considerable financial burden imposed on the health care system by the development of VAP.10,35,36,38–41
Microorganisms responsible for VAP differ according to the population of ICU patients, the durations of hospital and ICU stays, and the specific diagnostic method(s) used to establish the responsible pathogens. A number of studies have shown that gram-negative bacilli cause many of the respiratory infections in this setting.1,2,42,43 The data from twenty-four studies conducted on ventilated patients, for whom bacteriologic studies were restricted to uncontaminated specimens obtained using a protected specimen brush (PSB) or bronchoalveolar lavage (BAL), confirmed these results: gram-negative bacilli represented 58% of recovered organisms (Table 46-1).1 The predominant gram-negative bacilli were P. aeruginosa and Acinetobacter spp., followed by Proteus spp., Escherichia coli, Klebsiella spp., and H. influenzae. A relatively high rate of gram-positive pneumonias was also reported in those studies, with Staphylococcus aureus involved in more than 20% of the cases.42 Many episodes of VAP are caused by multiple pathogens.1,44
| Pathogen | Frequency (%) |
|---|---|
| Pseudomonas aeruginosa | 24.4 |
| Acinetobacter spp. | 7.9 |
| Stenotrophomonas maltophilia | 1.7 |
| Enterobacteriaceaea | 14.1 |
| Haemophilus spp. | 9.8 |
| Staphylococcus aureusb | 20.4 |
| Streptococcus spp. | 8.0 |
| Streptococcus pneumoniae | 4.1 |
| Coagulase-negative staphylococci | 1.4 |
| Neisseria spp. | 2.6 |
| Anaerobes | 0.9 |
| Fungi | 0.9 |
| Others (<1% each)c | 3.8 |
Underlying diseases may predispose patients to infection with specific organisms. Patients with chronic obstructive pulmonary disease are at increased risk for H. influenzae, Moraxella catarrhalis, or S. pneumoniae infections; cystic fibrosis increases the risk of P. aeruginosa and/or S. aureus infections, while trauma and neurologic disease increases the risk for S. aureus infection. Furthermore, the causative agent for pneumonia differs among ICU surgical populations, with 18% of the nosocomial pneumonias caused by Haemophilus or pneumococci, particularly in patients with trauma, but not in patients with malignancy or who underwent transplantation, abdominal, or cardiovascular surgery.1,2
Despite somewhat different definitions of early onset pneumonia, varying from onset of less than 3 days to less than 7 days, high rates of H. influenzae, S. pneumoniae, methicillin-susceptible Staphylococcus aureus (MSSA) or susceptible Enterobacteriaceae were constantly found in early onset VAP, whereas P. aeruginosa, Acinetobacter spp., methicillin-resistant Staphylococcus aureus (MRSA), and multiresistant gram-negative bacilli were significantly more frequent in late-onset VAP.1 The different pattern of distribution of etiologic agents between early- and late-onset VAP is linked to prior antimicrobial therapy in many patients with late-onset VAP. When multivariate analysis was used to identify risk factors for VAP caused by potentially drug-resistant bacteria such as MRSA, P. aeruginosa, Acinetobacter baumannii, and/or Stenotrophomonas maltophilia in 135 consecutive episodes of VAP, only three variables remained significant: mechanical ventilation duration of longer than 7 days before onset of VAP, prior antibiotic use, and prior use of broad-spectrum drugs (third-generation cephalosporins, fluoroquinolones, and/or imipenem).45 Not all studies have confirmed this distribution pattern, and in some studies the most common pathogens associated with early onset VAP were P. aeruginosa, MRSA, and Enterobacter spp., with similar pathogens associated with late-onset VAP.46,47 These findings might be explained in part by prior hospitalization and the use of antibiotics before transfer to the ICU.
The incidence of multiresistant pathogens is also closely linked to local factors and varies widely from one institution to another. Consequently, each ICU has to continuously collect meticulous epidemiologic data.48 Clinicians must clearly be aware of the common microorganisms associated with both early-onset and late-onset VAP in their own hospitals so as to avoid the administration of initial inadequate antimicrobial therapy.
Legionella species, anaerobes, and even Pneumocystis jiroveci should be mentioned as potential causative agents, but these microbes are not commonly found when pneumonia is acquired during mechanical ventilation. Herpesviridae, namely herpes simplex virus, can be detected in the lower respiratory tracts of 5% to 64% of ICU patients, depending on the population and the diagnostic method used. In most cases, herpes simplex virus recovery from lower respiratory tract samples of nonimmunocompromised ventilated patients corresponds to viral contamination from the mouth and/or throat. For some patients, however, real herpes simplex virus bronchopneumonitis can develop, and it can evolve into ARDS and/or facilitate the occurrence of bacterial superinfection.49–51 Cytomegalovirus-induced pneumonia is a rare event in ventilated patients. As for herpes simplex virus bronchopneumonitis, it is impossible to know whether cytomegalovirus detection in the lower respiratory tract is merely a marker of disease severity or signals real disease with its own morbidity and mortality.52–55
Isolation of fungi, most frequently Candida species, at significant concentrations poses interpretative problems. Invasive disease has been reported in VAP but yeasts are isolated more frequently from respiratory tract specimens in the absence of apparent disease, even when retrieved at high concentrations from bronchoscopic specimens.56–60 Thus, based on current data, the presence of yeasts in respiratory secretions obtained from nonimmunosuppressed ventilated patients usually indicates colonization rather than infection of the respiratory tract and does not justify by itself a specific antifungal therapy. Evidence of lung tissue invasion is needed for making the diagnosis of Candida pneumonia in such a setting. Interactions, however, between Candida and bacteria, particularly Pseudomonas, have been reported, and colonization of the respiratory tract by yeasts may predispose to bacterial VAP.61–64
By examining currently available data, the clinical significance of anaerobes in the pathogenesis and outcome of VAP remains unclear except as etiologic agents in patients with necrotizing pneumonitis, lung abscess, or pleuropulmonary infections. Anaerobic infection and coverage with antibiotics, such as clindamycin or metronidazole, should probably also be considered for patients with respiratory secretions documenting numerous extracellular and intracellular microorganisms after Gram staining in the absence of positive cultures for aerobic pathogens.
Pathogenesis and Predisposing Factors
Pneumonia results from microbial invasion of the normally sterile lower respiratory tract and lung parenchyma caused by either a defect in host defenses, a challenge by a particularly virulent microorganism, or an overwhelming inoculum. The normal human respiratory tract possesses a variety of defense mechanisms that protect the lung from infection. Examples include anatomic barriers, such as the glottis and larynx; cough reflexes; tracheobronchial secretions; mucociliary lining; cell-mediated and humoral immunity; and a dual phagocytic system that involves both alveolar macrophages and neutrophils.1 When these coordinated components function properly, invading microbes are eliminated and clinical disease is avoided. When these defenses are impaired, however, or if they are overcome by virtue of a high inoculum of organisms or organisms of unusual virulence, pneumonitis may result.
As suggested by the infrequent association of VAP with bacteremia, most of these infections appear to result from aspiration of potential pathogens that have colonized the mucosal surfaces of the oropharyngeal airways. Intubation of the patient not only compromises the natural barrier between the oropharynx and trachea, but may also facilitate the entry of bacteria into the lung by pooling and leakage of contaminated secretions around the endotracheal tube cuff.65 This phenomenon occurs in most intubated patients, whose supine position may facilitate its occurrence. In previously healthy, newly hospitalized patients, normal mouth flora or pathogens associated with community-acquired pneumonia may predominate. In sicker patients who have been hospitalized more than 5 days, gram-negative bacilli and S. aureus frequently colonize the upper airway.66
Uncommonly, VAP may arise in other ways. Observed “macroaspirations” of gastric material initiate the process in some patients. Allowing condensates in ventilator tubing to drain into the patient’s airway may have the same effect. Fiber-optic bronchoscopy, tracheal suctioning, or manual ventilation with contaminated equipment may also bring pathogens to the lower respiratory tract. More recently, concerns have focused on the potential role of contaminated inline medication nebulizers, but these devices are infrequently associated with VAP.
Risk factors for tracheobronchial colonization with gram-negative bacilli appear to be the same as those that favor pneumonia and include more severe illness, longer hospitalization, prior or concomitant use of antibiotics, malnutrition, intubation, azotemia, and underlying pulmonary disease.67 Experimental investigations have linked some of these risk factors to changes in adherence of gram-negative bacilli to respiratory epithelial cells. Although formerly attributed to losses of cell-surface fibronectin, these changes in adherence more likely reflect alterations of cell-surface carbohydrates. Bacterial adhesins and prior antimicrobial therapy appear to facilitate the process. Interestingly, Enterobacteriaceae usually appear in the oropharynx first, whereas P. aeruginosa more often appears first in the trachea.68
Other sources of pathogens causing VAP include the paranasal sinuses, dental plaque, and the subglottic area between the true vocal cords and the endotracheal tube cuff. Not all authors agree that the gastropulmonary route of infection is truly operative in ICU patients.69 Progression of colonization from the stomach to the upper respiratory tract with subsequent episodes of VAP could not be demonstrated in several studies and efforts to eliminate the gastric reservoir with antimicrobial therapy without decontaminating the oropharyngeal cavity have generally failed to prevent VAP.69–71 In fact, there is more than one potential pathway for colonization of the oropharynx and trachea in such a setting, including fecal–oral cross-infection on the hands of health care personnel, and contaminated respiratory therapy equipment. Patient-care activities, such as bathing, oral care, tracheal suctioning, enteral feeding, and the tube manipulations, provide ample opportunities for transmission of pathogens when infection-control practices are substandard.72
Risk factors provide information on the probability of lung infection developing in individuals and populations. Thus, they may contribute to the elaboration of effective preventive strategies by indicating which patients might be most likely to benefit from prophylaxis against pneumonia. Table 46-2 summarizes the independent factors for VAP that were identified by multivariate analyses in selected studies.13,25,38,73–80
| Host Factors | Intervention Factors |
|---|---|
|
|
Postsurgical patients are at increased risk for VAP. In a 1981 report, the pneumonia rate during the postoperative period was 17%.81 Those authors stated that the development of pneumonia was closely associated with preoperative markers of severity of the underlying disease, such as low serum albumin concentration and a high score on the American Society of Anesthesiologists preanesthesia physical status classification. A history of smoking, longer preoperative stays, longer surgical procedures and thoracic or upper abdominal surgery were also significant risk factors for postsurgical pneumonia. Another study comparing adult ICU populations demonstrated that postoperative patients had consistently higher rates of nosocomial pneumonia than did medical ICU patients, with a risk ratio of 2.2.79 Multiple regression analysis was performed to identify independent predictors of nosocomial pneumonia in the two groups; for surgical ICU patients, mechanical ventilation (>2 days) and Acute Physiology and Chronic Health Evaluation (APACHE) score were retained by the model; for the medical ICU population, only mechanical ventilation (>2 days) remained significant. It has been suggested that different surgical ICU patient populations may have different risks for nosocomial pneumonia: Cardiothoracic surgery and trauma (particularly head trauma) patients were more likely to develop VAP than medical or other types of surgical patients.13
The use of antibiotics in the hospital setting is associated with an increased risk of nosocomial pneumonia and selection of resistant pathogens.22,25,45,66,82–85 In a cohort study of 320 patients, prior antibiotic administration was identified by logistic regression analysis to be one of the four variables independently associated with VAP along with organ failure, age greater than 60 years, and the patient’s head positioning (i.e., flat on the patient’s back or supine vs. head and thorax raised 30 degrees to 40 degrees or semirecumbent).22 Other investigators, however, found that antibiotic administration during the first 8 days was associated with a lower risk of early onset VAP.86 For example, Sirvent et al showed that a single dose of a first-generation cephalosporin given prophylactically was associated with a lower rate of early-onset VAP in patients with structural coma.87 Moreover, multiple logistic regression analysis of risk factors for VAP in 358 medical ICU patients identified the absence of antimicrobial therapy as one of the factors independently associated with VAP onset.88 Finally, the results of the multicenter Canadian study on the incidence of and risk factors for VAP indicated that antibiotic treatment conferred protection against VAP.13 This apparent protective effect of antibiotics disappears after 2 to 3 weeks, suggesting that a higher risk of VAP cannot be excluded beyond this point. Thus, risk factors for VAP change over time, thereby explaining why they differ from one series to another.
In theory, patients receiving stress-ulcer prophylaxis that does not change gastric acidity, such as sucralfate, should have lower rates of gastric bacterial colonization and, consequently, a lower risk for nosocomial pneumonia, than those receiving antacids or H2 blockers.89–90
According to meta-analyses of the efficacy of stress-ulcer prophylaxis in ICU patients, respiratory tract infections were significantly less frequent in patients treated with sucralfate than those receiving antacids or H2 blockers.91,92 This conclusion, however, was not fully confirmed in a very large, multicenter, randomized, blinded, placebo-controlled trial that compared sucralfate suspension (1 g every 6 hours) with the H2-receptor antagonist ranitidine (50 mg every 8 hours) for the prevention of upper gastrointestinal bleeding in 1200 ventilated patients.93 Clinically relevant gastrointestinal bleeding developed in ten of the 596 (1.7%) patients receiving ranitidine, as compared with twenty-three of the 604 (3.8%) receiving sucralfate (relative risk [RR], 0.44; 95% confidence interval [CI], 0.21 to 0.92; p = 0.02). In the ranitidine group, 114 of 596 (19.1%) patients had VAP, as diagnosed by an adjudication committee using a modified version of the Centers for Disease Control and Prevention criteria, versus ninety-eight of 604 (16.2%) in the sucralfate group (RR, 1.18; 95% CI, 0.92 to 1.51; p = 0.19). VAP, however, occurred significantly less frequently in patients receiving sucralfate when the diagnosis of pneumonia was based on Memphis VAP Consensus Conference criteria (if there was radiographic evidence of abscess and a positive needle aspirate, or histologic proof of pneumonia at biopsy or autopsy) (p = 0.03).93
Sucralfate appears to have a small protective effect against VAP because stress-ulcer prophylactic medications that raise the gastric pH might themselves increase the incidence of pneumonia.94,95 This contention is supported by direct comparisons of trials of H2-receptor antagonists versus no prophylaxis, which showed a trend toward higher pneumonia rates among the patients receiving H2-receptor antagonists (odds ratio [OR], 1.25; 95% CI, 0.78 to 2.00).91 Furthermore, the comparative effects of sucralfate and no prophylaxis are unclear. Among 226 patients enrolled in two randomized trials, those receiving sucralfate tended to develop pneumonia more frequently than those given no prophylaxis (OR, 2.11; 95% CI, 0.82 to 5.44).96,97
The presence of an endotracheal tube by itself circumvents host defenses, causes local trauma and inflammation, and increases the probability of aspiration of nosocomial pathogens from the oropharynx around the cuff. Scanning electron microscopy of 25 endotracheal tubes revealed that 96% had partial bacterial colonization and 84% were completely coated with bacteria in a biofilm or glycocalyx.98 The authors hypothesized that bacterial aggregates in biofilm dislodged during suctioning might not be killed by antibiotics or effectively cleared by host immune defenses. Clearly, the type of endotracheal tube may also influence the likelihood of aspiration. Use of low-volume, high-pressure endotracheal cuffs reduced the rate to 56% and the advent of high-volume, low-pressure cuffs further lowered it to 20%.99 Leakage around the cuff allows secretions pooled above the cuff to enter the trachea; this mechanism, recently confirmed, underlines the importance of maintaining adequate intracuff pressure for preventing VAP.100
In addition to the presence of endotracheal tubes, reintubation is, per se, a risk factor for VAP.101 This finding probably reflects an increased risk of aspiration of colonized oropharyngeal secretions into the lower airways by patients with subglottic dysfunction or impaired consciousness after several days of intubation. Another explanation is direct aspiration of gastric contents into the lower airways, particularly when a nasogastric tube is kept in place after extubation.
Some investigators postulated that early tracheotomy could lower VAP rate because it can permit easier oral hygiene and bronchopulmonary toilet or less time spent deeply sedated.102 Such benefit, however, was not confirmed in other studies, including two large recent randomized trials having systematically evaluated this issue.103–106
Almost all ventilated patients have a nasogastric tube inserted to evacuate gastric and enteral secretions, prevent gastric distension, and/or provide nutritional support. The nasogastric tube is not generally considered to be a potential risk factor for VAP, but it may increase oropharyngeal colonization, cause stagnation of oropharyngeal secretions, and increase reflux and the risk of aspiration. A multivariate analysis retained the presence of a nasogastric tube as one of the three independent risk factors for nosocomial pneumonia based on a series of 203 patients admitted to the ICU for 72 hours or more.77
Early initiation of enteral feeding is generally regarded as beneficial in critically ill patients, but it may increase the risk of gastric colonization, gastroesophageal reflux, aspiration, and pneumonia.107,108 The aspiration rate generally varies as a function of differences in the patient population, neurologic function, type of feeding tube, location of the feeding port, and method of evaluating aspiration. Clinical impressions and preliminary data suggest that postpyloric or jejunal feeding entails less risk of aspiration and may therefore be associated with fewer infectious complications than gastric feeding, although this point remains controversial.109,110 Nonetheless, aspiration can easily occur should the feeding tube be inadvertently dislodged. A retrospective study of non–critically ill adult patients showed a 40% rate of accidental feeding tube dislodgment, but all the patients whose tube was dislodged were confused or disoriented or had altered awareness, as is frequently observed in ICU patients.111
Maintaining ventilated patients with a nasogastric tube in place in a supine position is also a risk factor for aspiration of gastric contents into the lower airways. When radioactive material was injected through a nasogastric tube directly into the stomach of nineteen ventilated patients, the mean radioactive counts in endobronchial secretions were higher in a time-dependent fashion in samples obtained from patients in a supine position than in those obtained from patients in a semirecumbent position.112 The same microorganisms were isolated from the stomach, pharynx, and endobronchial samples of 32% of the specimens taken while patients were lying supine. The same investigators conducted a randomized trial comparing semirecumbent and supine positions.113 The trial, which included eighty-six intubated and ventilated patients, was stopped after the planned interim analysis because the frequency and the risk of VAP were significantly lower for the semirecumbent group. These findings were indirectly confirmed by the demonstration that the head position of the supine patient during the first 24 hours of mechanical ventilation was an independent risk factor for acquiring VAP.22 To what degree of elevation, however, the head of bed should be targeted remains controversial.114–117
Ventilators with humidifying cascades often have high levels of tubing colonization and condensate formation that may also be risk factors for pneumonia. The rate of condensate formation in the ventilator circuit is linked to the temperature difference between the inspiratory-phase gas and the ambient temperature, and may be as high as 20 to 40 mL/hour.118,119 Examination of condensate colonization in twenty circuits detected a median level of 2.0 × 105 organisms/mL, and 73% of the fifty-two gram-negative isolates present in the patients’ sputum samples were subsequently isolated from condensates.119 Because most of the tubing colonization was derived from the patients’ secretions, the highest bacterial counts were present near the endotracheal tube. Simple procedures, such as turning the patient or raising the bed rail, may accidentally spill contaminated condensate directly into the patient’s tracheobronchial tree.120 Inoculation of large amounts of fluid with high bacterial concentrations is an excellent way to overwhelm pulmonary defense mechanisms and cause pneumonia. Heating ventilator tubing markedly lowers the rate of condensate formation, but heated circuits are often nondisposable and are expensive. Inline devices with one-way valves to collect the condensate are probably the easiest way to handle this problem; they must be correctly positioned into disposable circuits and emptied regularly.
To decrease condensation and moisture accumulation in ventilator circuits, several studies have investigated the use of heat–moisture exchangers in place of conventional heated-water humidification systems. Slightly lower VAP rates were observed in four studies and a significant difference in a fifth study, suggesting that heat–moisture exchangers are at least comparable to heated humidifiers and may be associated with lower VAP rates than heated humidifiers.121–125 Changing the heat–moisture exchangers every 48 hours did not affect ventilator circuit colonization and the authors concluded that the cost of mechanical ventilation might be substantially reduced without any detriment to the patient by prolonging the time between heat–moisture exchangers changes from 24 to 48 hours.126 Furthermore, using heat–moisture exchangers may decrease the nurses’ workload (no need to refill cascades, to void water traps on circuits, and so on), decrease the number of septic procedures (it was clearly shown that respiratory tubing condensates must be handled as an infectious waste), and reduce the cost of mechanical ventilation, especially when used for prolonged periods without change. Because some observational studies, however, have documented an increased resistive load and a larger dead space associated with exchangers,127,128 their use should be discouraged in patients with ARDS ventilated with a low tidal volume and in patients with chronic obstructive pulmonary disease during the weaning period, if pressure support, and not T-piece trials, are used.
There is no apparent advantage to changing ventilator circuits frequently for VAP prevention. This holds true whether circuits are changed every 2 days or every 7 days compared with no change at all, and whether they are changed weekly as opposed to three times per week.129–131 A policy of no circuit changes or infrequent circuit changes is simple to implement and the costs are likely lower than those generated by regular, frequent circuit changes; thus, such a policy is strongly recommended by the 1997 Centers for Disease Control and Prevention guidelines and other guidelines.132–134
While many studies have compared the risk of nosocomial sinusitis as a function of the intubation method used and the associated risk of VAP, only a few were adequately powered to give a clear answer. In one study of 300 patients who required mechanical ventilation for at least 7 days and were randomly assigned to undergo nasotracheal or orotracheal intubation, computed tomographic evidence of sinusitis was observed slightly more frequently in the nasal than oral endotracheal group (p = 0.08), but this difference disappeared when only bacteriologically confirmed sinusitis was considered.135
The rate of infectious maxillary sinusitis and its clinical relevance were also prospectively studied in 162 consecutive critically ill patients, who had been intubated and ventilated for 1 hour to 12 days before enrollment.136 All had a paranasal computed tomography scan within 48 hours of admission, which was used to divide them into three groups (no, moderate, or severe sinusitis), according to the radiologic appearance of the maxillary sinuses. Patients who had no sinusitis at admission (n = 40) were randomized to receive endotracheal and gastric tubes via the nasal or oral route and, based on radiologic images, respective sinusitis rates were 96% and 23% (p < 0.03); yet, no differences in the rates of infectious sinusitis were documented according to the intubation route. VAP, however, was more common in patients with infectious sinusitis, with 67% of them developing lung infection in the days following the diagnosis of sinusitis.136 Therefore, although it seems clear that infectious sinusitis is a risk factor for VAP, no studies have yet been able to definitively demonstrate that orotracheal intubation decreases the infectious sinusitis rate compared to nasotracheal intubation. Thus, no firm recommendations on the best route of intubation to prevent VAP can be advanced.
A prospective cohort study conducted in 531 ventilated patients evaluated the impact of transporting the patient out of the ICU to other sites within the hospital.137 Results showed that 52% of the patients had to be moved at least once, for a total of 993 transports, and that 24% of the transported patients developed VAP compared with 4% of the patients confined to the ICU (p < 0.001). Multiple logistic regression analysis confirmed that transport out of the ICU was independently associated with VAP (OR = 3.8; p < 0.001).
Diagnosis
VAP is typically suspected when a patient has new or progressive radiographic infiltrates and clinical findings suggesting infection, such as the new onset of fever, purulent sputum, leukocytosis, increased minute ventilation, and/or a decline in arterial oxygenation. Because interpretation of chest radiographs is difficult, particularly in patients with prior abnormalities, such as ARDS, it is also mandatory to consider the diagnosis of VAP in ventilated patients who clinically deteriorate, and/or in whom vasopressors should be increased to maintain blood pressure, even in the absence of a clear-cut progression of the radiographic abnormalities.
The systemic signs of infection, however, such as fever, tachycardia, and leukocytosis, are nonspecific findings that can be caused by any condition that releases cytokines. In trauma and other surgical patients, fever and leukocytosis should prompt the physician to suspect infection, but during the early posttraumatic or postoperative period (i.e., during the first 72 hours), these findings usually are not conclusive. Later, fever and leukocytosis are more likely to be caused by pulmonary or nonpulmonary (vascular catheter infection, gastrointestinal infection, urinary tract infection, sinusitis, or wound infection) infections, but even then, other events associated with an inflammatory response (e.g., devascularized tissue, open wounds, pulmonary edema, and/or infarction) can be responsible for these findings. Although the plain (usually portable) chest roentgenogram remains an important component in the evaluation of ventilated patients with suspected pneumonia, it is most helpful when it is normal and rules out pneumonia. When infiltrates are evident, the particular pattern is of limited value for differentiating among cardiogenic pulmonary edema, noncardiogenic pulmonary edema, pulmonary contusion, atelectasis (or collapse), and pneumonia, even when using computed tomographic scanning.8,17,138–142 Because the tracheobronchial tree of mechanically ventilated patients is frequently rapidly colonized by potential pathogens, the presence of bacteria at that level is not a sufficient argument to diagnose true lung infection, which constitutes another major obstacle for the diagnosis of VAP.8,143
In 1991, a composite clinical score, the clinical pulmonary infection score (CPIS) was proposed, based on seven variables (temperature, blood leukocyte count, volume and purulence of tracheal secretions, oxygenation, pulmonary radiography, and semiquantitative culture of tracheal aspirate) accorded 0, 1, or 2 points.144 This scoring system, however, is quite tedious to calculate and difficult to use in clinical practice, because several variables, such as progression of pulmonary infiltrates and results of semiquantitative cultures of tracheal secretions, can lead to different calculations depending on the observer.145 Furthermore, its value was not validated in several subsequent prospective studies, especially in patients with bilateral pulmonary infiltrates.146–154
Thus, as soon as a ventilated patient is suspected of developing pneumonia, a more complete diagnostic workup should be undertaken, targeting two objectives. The first objective is the immediate recognition of a true VAP or of an extrapulmonary bacterial infection, so as to start effective antibiotics against the microorganisms responsible for infection as soon as possible.1,2 Numerous studies indicate that failure to initiate prompt appropriate antimicrobial treatment in this setting is a major risk factor for an increased morbidity and mortality.155–163 The second objective is to avoid overusing antibiotics in patients with only proximal airways colonization and no ongoing bacterial infection. Epidemiologic investigations have clearly demonstrated that indiscriminate use of antimicrobial agents in ICU patients may have immediate and long-term consequences, which contribute to emergence of multiresistant pathogens and increase the risk of serious superinfections.164–169 This risk is not limited to one patient. Instead, the risk of colonization or infection by multidrug-resistant strains is increased in patients throughout the ICU and even the entire hospital. Virtually all reports emphasize that better antibiotic control programs to limit bacterial resistance are urgently needed in ICUs, and that patients without true infection should not receive antimicrobial treatment.164
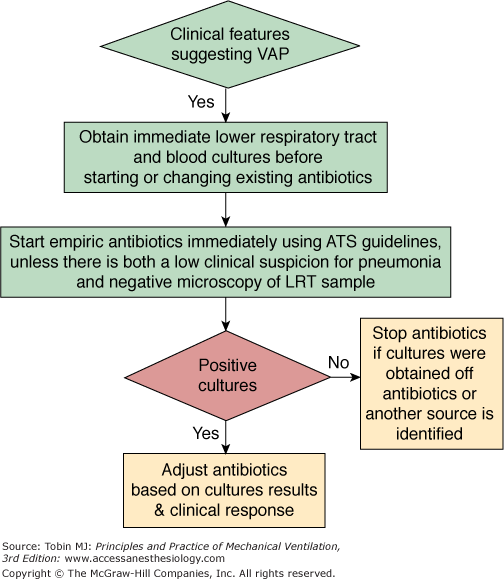
To reach these objectives, all diagnostic strategies should follow three consecutive steps: (a) obtaining a respiratory tract sample (from proximal or distal airways) for microscopy and culture (qualitative, semiquantitative, or quantitative) before introduction of new antibiotics; (b) immediately starting empiric antimicrobial treatment, unless there are both a negative microscopy and no signs of severe sepsis; and (c) reevaluating treatment on day 2 or 3, based on microbiologic cultures results and clinical outcome.1,2
The first option is to use a clinical strategy and to treat every patient clinically suspected of having a pulmonary infection with new antibiotics (even when the likelihood of infection is low), arguing that several studies showed that immediate initiation of appropriate antibiotics was associated with reduced mortality.28,32,157,170–175 Using this strategy, all patients suspected of having VAP are treated with new antibiotics after having obtaining an endotracheal aspirate for microscopy and qualitative culture. The selection of appropriate empirical therapy is based on risk factors and local microbiologic and resistance patterns, and involves qualitative testing to identify possible pathogens. The initial antimicrobial therapy is adjusted according to culture results and clinical response (Fig. 46-1). Antimicrobial treatment is discontinued if and only if the following three criteria are fulfilled on day 3: (a) clinical diagnosis of VAP is unlikely (there are no definite infiltrates found on chest radiography at follow-up and no more than one of the three following findings are present: temperature higher than 38.3°C [100.9°F], leukocytosis or leukopenia, and purulent tracheobronchial secretions) or an alternative noninfectious diagnosis is confirmed; (b) tracheobronchial aspirate culture results are nonsignificant; and (c) severe sepsis or shock are not present.176
This clinical approach has two undisputable advantages: first, no specialized microbiologic techniques are required, and, second, the risk of missing a patient who needs antimicrobial treatment is minimal when all suspected patients are treated with new antibiotics. Because, however, tracheobronchial aspirate culture results are rarely negative secondary to the high rate of proximal airways colonization observed in patients receiving mechanical ventilation, discontinuation of antibiotics on day 3 is difficult to perform, leading to antibiotic overuse in many ICU patients. Qualitative endotracheal aspirate cultures contribute indisputably to the diagnosis of VAP only when they are completely negative for a patient with no modification of prior antimicrobial treatment. In such a case, the negative-predictive value is very high and the probability of the patient having pneumonia is close to zero.5 This is why some investigators have proposed to replace qualitative cultures of endotracheal aspirates by semiquantitative or quantitative cultures of the same specimens.177
Several studies using quantitative culture techniques suggest that endotracheal aspirate cultures may have an acceptable overall diagnostic accuracy, similar to that of several other more invasive techniques.177 Not all studies, however, have confirmed this conclusion. To assess the reliability of that method, bronchoscopy with PSB and BAL was used to study fifty-seven episodes of suspected lung infection in thirty-nine ventilator-dependent patients with no recent changes of antimicrobial therapy.178 The operating characteristics of endotracheal aspirate cultures were calculated over a range of cutoff values (from 103 to 107 colony-forming units [CFU]/mL); the threshold of 106 CFU/mL appeared to be the most accurate, with a sensitivity of 68% and a specificity of 84%. When this threshold was applied to the study population, however, almost one-third of the patients with pneumonia were not identified. Furthermore, only 40% of microorganisms cultured in endotracheal aspirate samples coincided with those obtained from PSB specimens. Other authors have emphasized that, although quantitative endotracheal aspirate cultures can correctly identify patients with pneumonia, microbiologic results cannot be used to infer which microorganisms present in the trachea are really present in the lungs. In a study comparing quantitative endotracheal aspirate culture results to postmortem quantitative lung biopsy cultures, only 53% of the microorganisms isolated from the former samples at concentrations greater than 107 CFU/mL were also found in the latter cultures.179
The inherent advantage of quantitative cultures of endotracheal aspirates is that they are more specific, permitting the discontinuation of antibiotics in more patients than when using only qualitative cultures. But it must be kept in mind that this technique has several potential pitfalls. First, many patients may not be identified using the cutoff value of 106 CFU/mL. Second, as soon as a lower threshold is used, specificity declines sharply and overtreatment becomes a problem. Finally, selecting antimicrobial therapy solely on the basis of endotracheal aspirate culture results can lead to either unnecessary antibiotic therapy or overtreatment with broad-spectrum antimicrobial agents.
Another option when using the clinical approach is to follow the strategy described by Singh et al, in which decisions regarding initial antibiotic therapy are based on CPIS, a clinical score constructed from seven variables.180 Patients with CPIS greater than 6 are treated as having VAP with antibiotics for 10 to 21 days, and antibiotics are discontinued after 3 days if the CPIS is 6 or less (Fig. 46-2). Such an approach avoids prolonged treatment of patients who have a low likelihood of infection, while allowing immediate treatment of patients who are more likely to have VAP.
Figure 46-2
Diagnostic and therapeutic strategy applied to patients managed with the strategy proposed by Singh et al.180
Two conditions must be fulfilled when using this strategy. First, the selection of initial antimicrobial therapy should be based on the most common microbes responsible for VAP at each institution. For example, ciprofloxacin would not be the right choice in hospitals with a high prevalence of MRSA infections. Second, physicians should reevaluate antimicrobial treatment on day 3, when susceptibility patterns of the microorganism(s) recovered from pulmonary secretions are available, so as to select treatment with a narrower spectrum antibiotic.
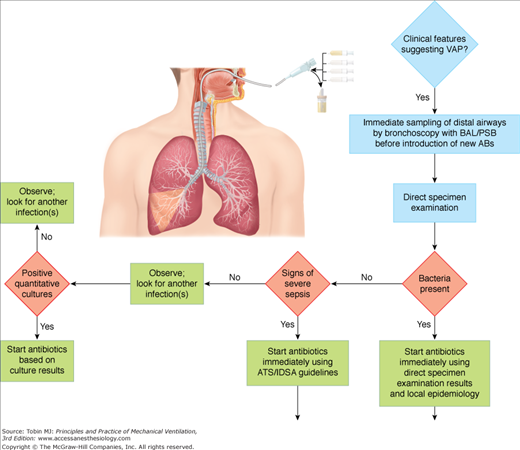
This strategy uses quantitative cultures of lower respiratory secretions (BAL or PSB collected with a bronchoscope) to define both the presence of pneumonia and the etiologic pathogen(s). Pathogens are present in inflammatory secretions of the lower respiratory tract at concentrations of at least 105 to 106 CFU/mL, whereas contaminants are generally present at less than 104 CFU/mL.181 The diagnostic thresholds proposed for PSB and BAL are based on this concept. Because PSB collects between 0.001 and 0.01 mL of secretions, the presence of greater than 103 bacteria in the originally diluted sample (1 mL) actually represents 105 to 106 CFU/mL of pulmonary secretions. Similarly, 104 CFU/mL for BAL, which collects 1 mL of secretions in 10 to 100 mL of effluent, represents 105 to 106 CFU/mL.182–184 Using this strategy, therapeutic decisions are tightly protocolized, using the results of direct examination of distal pulmonary samples and results of quantitative cultures in deciding whether to start antibiotic therapy, which pathogens are responsible for infection, which antimicrobial agents to use, and whether to continue therapy (Fig. 46-3).
One major technical problem with all bronchoscopic techniques is proper selection of the sampling area in the tracheobronchial tree. Almost all intubated patients have purulent-looking secretions and the secretions first seen may represent those aspirated from another site into gravity-dependent airways or from upper-airway secretions aspirated around the endotracheal tube. Usually, the sampling area is selected based on the location of infiltrate on chest radiograph or the segment visualized during bronchoscopy as having purulent secretions.185 Collection of secretions in the lower trachea or mainstem bronchi, which may represent recently aspirated secretions around the endotracheal tube cuff, should be avoided. In patients with diffuse pulmonary infiltrates or minimal changes in a previously abnormal chest radiograph, determining the correct airway to sample may be difficult. In these cases, sampling should be directed to the area where endobronchial abnormalities are maximal.186 In case of doubt, and because autopsy studies indicate that VAP frequently involves the posterior portion of the right lower lobe, this area should probably be sampled as a priority.187 Although bilateral sampling in the immunosuppressed host with diffuse infiltrates has been advocated, there is no convincing evidence that multiple specimens are more accurate than single specimens for diagnosing nosocomial bacterial pneumonia in ventilated patients.
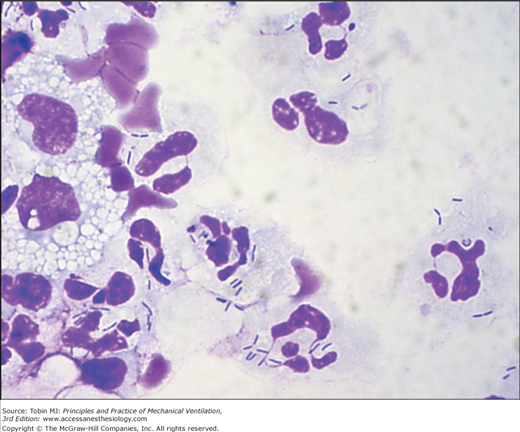
Because BAL harvests of cells and secretions from a large area of the lung and specimens can be microscopically examined immediately after the procedure to detect the presence or absence of intracellular or extracellular bacteria in the lower respiratory tract, it is particularly well suited to provide rapid identification of patients with pneumonia (Figs. 46-4 and 46-5).183,188–193 Assessment of the degree of qualitative agreement between Gram stains of BAL fluid and PSB quantitative cultures for a series of fifty-one patients with VAP, however, showed correspondence to be complete for 51%, partial for 39%, and nonexistent for 10% of the cases.190
Many groups have investigated the value of quantitative BAL culture for the diagnosis of pneumonia in ICU patients.183,194,195 When the results of the eleven studies evaluating BAL fluids from a total of 435 ICU patients with nosocomial pneumonia were pooled, overall accuracy was very close to that of PSB: The Q value was 0.84 (Q represents the intersection between the summary receiver operating characteristics curve and a diagonal from the upper-left corner to the lower-right corner of the receiver operating characteristics space).194 Similar conclusions were drawn in another meta-analysis, which pooled the results of twenty-three studies: Sensitivity and specificity of BAL were 73% ± 18% and 82% ± 19%, respectively.195 When analysis in these studies was restricted to patients without prior antibiotics or when only lung tissue cultures were used as the reference standard, results of bronchoscopic techniques pneumonia were much better: Sensitivity was always greater than 80%.
Other studies confirm the accuracy of bronchoscopic techniques for diagnosing nosocomial pneumonia. In a study evaluating spontaneous lung infections occurring in ventilated baboons with permeability pulmonary edema, Johanson et al found excellent correlation between the bacterial content of lung tissue and results of quantitative culture of lavage fluid.196 BAL recovered 74% of all species present in lung tissue, including 100% of species present at a concentration equal to or greater than 104 CFU/g of tissue. Similarly, in twenty ventilated patients who had not developed pneumonia before the terminal phase of disease and who had no recent changes in antimicrobial therapy, Chastre et al found that bronchoscopic BAL specimens obtained just after death identified 90% of all species present in the lung, with a strong correlation between the results of quantitative cultures of both specimens.183 These findings confirm that bronchoscopic BAL samples very reliably identify, both qualitatively and quantitatively, microorganisms present in lung segments, even when the pneumonia develops as a superinfection in a patient already receiving antimicrobial treatment for several days.
Values within 1 log10 of the cutoff must, however, be interpreted cautiously, and bronchoscopy should be repeated in symptomatic patients with a negative (<104 CFU/mL) result.197 Many technical factors, including medium and adequacy of incubation, and antibiotic or other toxic components, may influence results. Reproducibility of PSB sampling has been recently evaluated by three groups.198–200 Although in vitro repeatability was excellent and in vivo qualitative recovery 100%, quantitative results were more variable. In 14% to 17% of patients, results of replicate samples fell on both sides of the 103 CFU/mL threshold, and results varied by more than 1 log10 in 59% to 67% of samples.198–200 This variability is presumably related to both the irregular distribution of organisms in secretions and the very small volume actually sampled by PSB. As with all diagnostic tests, borderline PSB and/or BAL quantitative culture results should be interpreted cautiously, and the clinical circumstances should be considered before reaching any therapeutic decision.
The most compelling argument for invasive techniques coupled with quantitative cultures of PSB or BAL specimens is that they can reduce excessive antibiotic use. There is little disagreement that the clinical diagnosis of nosocomial pneumonia is overly sensitive and leads to the unnecessary use of broad-spectrum antibiotics. Because bronchoscopic techniques may be more specific, their use would reduce antibiotic pressure in the ICU, thereby limiting the emergence of drug-resistant strains and the attendant increased risks of superinfection.22,201 When culture results are available, BAL and/or PSB techniques facilitate precise identification of the offending organisms and their susceptibility patterns. Such data are invaluable for optimal antibiotic selection in patients with a true VAP. They also increase the confidence and comfort level of health care workers in managing patients with suspected nosocomial pneumonia.202 The more targeted use of antibiotics also could reduce overall costs, despite the expense of bronchoscopy and quantitative cultures, and minimize antibiotic-related toxicity. This is particularly true in patients who have late-onset VAP, in whom expensive combination therapy is commonly recommended. A conservative cost analysis in a trauma ICU suggested that the discontinuation of antibiotics upon the return of negative bronchoscopic quantitative culture results could lead to a savings of more than $1700 per patient suspected of VAP.203
Finally, a major benefit of a negative bronchoscopy is to direct attention away from the lungs as the source of fever. Many hospitalized patients with negative bronchoscopic cultures have other potential sites of infection that can be identified via a simple diagnostic protocol. In fifty patients with suspected VAP who underwent a systematic diagnostic protocol designed to identify all potential causes of fever and pulmonary densities, Meduri et al confirmed that lung infection was present in only 42% of cases; the frequent occurrence of multiple infectious and noninfectious processes justifies a systematic search for the source of fever in this setting.141 Delay in diagnosis or definitive treatment of the true site of infection may lead to prolonged antibiotic therapy, more antibiotic-associated complications, and induction of further organ dysfunction.204
At least fifteen studies have described a variety of nonbronchoscopic techniques using various types of endobronchial catheters for sampling distal lower respiratory tract secretions; globally, results have been similar to those obtained with bronchoscopy.205 Compared to conventional PSB and/or BAL, nonbronchoscopic techniques are less invasive, can be performed by clinicians not qualified to perform bronchoscopy, have lower initial costs than bronchoscopy, avoid potential contamination by the bronchoscopic channel, are associated with less compromise of gas exchange during the procedure, and can be performed even in patients intubated with small endotracheal tubes. Disadvantages include the potential sampling errors inherent in a blind technique and the lack of airway visualization. Although autopsy studies indicate that pneumonia in ventilator-dependent patients has often spread into every pulmonary lobe and predominantly involves the posterior portion of the lower lobes, several clinical studies on ventilated patients with pneumonia contradict those findings, as some patients had sterile cultures of PSB specimens from the noninvolved lung.17,206 Furthermore, although the authors of most studies concluded that the sensitivities of nonbronchoscopic and bronchoscopic techniques were comparable, the overall concordance was only approximately 80%, emphasizing that, in some patients, the diagnosis could be missed by a blind technique, especially in the case of pneumonia involving the left lung.17
Performing microbiologic cultures of pulmonary secretions for diagnostic purposes after initiation of new antibiotic therapy in patients suspected of having developed VAP leads to a high rate of false-negative results, regardless of the method of obtaining the secretions. In fact, all microbiologic techniques are of limited value in patients with a recent infiltrate who have received new antibiotics, even if they have received the new antibiotics for less than 24 hours. A negative finding could indicate that the patient has been successfully treated for pneumonia and the bacteria are eradicated, or that the patient had no lung infection to begin with. Using both PSB and BAL, Souweine et al prospectively investigated sixty-three episodes of suspected VAP.207 If patients had been treated with antibiotics but did not have a recent change in antibiotic class, sensitivity of PSB and BAL culture (83% and 77%, respectively) were similar to the sensitivities achieved in patients not being treated with antibiotics. In other words, prior therapy did not reduce the yield of diagnostic testing among patients receiving current antibiotics given to treat a prior infection. Conversely, if therapy was recent, sensitivity of invasive diagnostic methods, using traditional thresholds, was only 38% with BAL and 40% with PSB.207 These two clinical situations should be clearly distinguished before interpreting the results of pulmonary secretion cultures, irrespective of how they were obtained. In the second situation, when the patient receives new antibiotics after the appearance of signs suggesting VAP, no conclusion concerning the presence or absence of pneumonia can be drawn if culture results are negative.207–209 Pulmonary secretions therefore need to be obtained before starting new antibiotics, as is the case for all types of microbiologic samples.
Procalcitonin (PCT), a 116-amino-acid peptide that is one of the precursors of the hormone calcitonin, has been described as a good diagnostic marker of bacterial infection in patients with community-acquired infections, especially in patients with lower respiratory tract infection.210–213 Moreover, several interventional trials have shown that PCT could be used to start or to postpone antibiotic treatment in community-acquired lower respiratory tract infections.214–218 In patients with nosocomial infections, and particularly in patients with VAP, its usefulness as a diagnostic marker is more doubtful.219–223 There are several reasons to explain why PCT is not a good diagnostic marker in patients with suspected VAP. First, pneumonia may be a localized infection; thus, as for other localized infections, PCT can be synthesized locally without systemic release, explaining its low serum level or apparent decrease in patients with true pulmonary infections. Second, ICU patients may suffer from previous severe sepsis or septic shock, multiorgan failure, or may have developed a systemic inflammatory response syndrome after surgery or trauma, conditions known to increase blood level of biomarkers including PCT in the absence of infection.222 Thus, a high level of PCT the day VAP is suspected is not useful, because it is not possible to distinguish an elevation caused by a previous noninfectious condition from an elevation caused by an active infection. Third, it is known that a time lag of 24 to 48 hours can exist between bacterial infection onset and peak PCT release, and that may also explain the apparent low level of PCT on the day of VAP onset. Incorporating PCT values in clinical score (such as CPIS) did not improve its diagnostic value.221,222
The soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) molecule is known to be specifically released during several infectious processes.224 Although it was apparently a reliable marker of pneumonia, especially VAP, more recent studies obtained contradictory findings, thereby raising doubt as to its usefulness for VAP diagnosis.220,225–227 Pending additional studies, and because this marker is not routinely available, sTREM-1 is not recommended as an indicator to guide antibiotic use in such situations.
Gram-negative bacilli cause more than 80% of VAP episodes and are associated with high mortality. Because gram-negative bacilli pneumonia might be diagnosed more rapidly by endotoxin measurement in BAL fluid, several investigators tested this hypothesis.228–231 Applying a threshold of greater than 5 endotoxin units (EU/mL) in BAL fluid yielded the best operating characteristics for gram-negative bacilli pneumonia diagnosis (100% sensitivity; 75% specificity; area under the receiver operating characteristics curve: 0.88) in a series of sixty-three hospitalized adults suspected of having lung infection.229 Three other studies confirmed the potential contribution of this tool.228,230,231 These findings suggest that endotoxin determination in BAL fluid might become an acceptable adjunct for the rapid diagnosis of gram-negative bacilli pneumonia in the near future, when it will be available at bedside.
Aside from decision-analysis studies232,233 and a single retrospective study,202 five trials have used a randomized scheme to assess the effect of a diagnostic strategy on antibiotic use and outcome in patients suspected of having VAP.26,27,234–236 In three randomized studies conducted in Spain, no differences in mortality and morbidity were found when either invasive (PSB and/or BAL) or noninvasive (quantitative endotracheal aspirate cultures) techniques were used to diagnose VAP.26,27,234 These studies were relatively small, ranging from fifty-one to eighty-eight patients. Antibiotics were continued in all patients despite negative cultures, thereby offsetting the potential advantage of the specific diagnostic test in patients with suspected VAP. Several prospective studies have concluded that antibiotics can be stopped in patients with negative quantitative cultures, without adversely affecting the recurrence of pneumonia and mortality.188,237,238
In a French study in which 413 patients were randomized, those receiving bacteriologic management using BAL and/or PSB had a lower mortality rate on day 14, lower sepsis-related organ failure assessment scores on days 3 and 7, and less antibiotic use.235 Pertinently, twenty-two nonpulmonary infections were diagnosed in the bacteriologic strategy group and only five in the clinical strategy group, suggesting that overdiagnosis of VAP can lead to errors in identifying nonpulmonary infections. A randomized trial conducted by the Canadian Critical Care Trials Group investigated the effect of different diagnostic approaches on outcomes of 740 patients suspected of having VAP.236 There was no difference in the 28-day mortality rate in patients in whom BAL was used versus those in whom endotracheal aspiration was used as the diagnostic strategy. The BAL group and the endotracheal aspiration group also had similar rates of targeted antibiotic therapy on day 6, days alive without antibiotics, and maximum organ-dysfunction scores. Unfortunately, information about how the decision algorithms were followed in the two diagnostic arms once cultures were available was not provided, raising uncertainties about how de-escalation of antibiotic therapy was pursued in patients with negative BAL cultures. Obviously, the potential benefit of using a diagnostic tool such as BAL for safely restricting unnecessary antimicrobial therapy in such a setting can only be obtained when decisions regarding antibiotics are closely linked to bacteriologic results, including both direct examination and cultures of respiratory specimens.
Our personal bias is that use of bronchoscopic techniques to obtain PSB and BAL specimens from an affected area of the lung in ventilated patients with signs suggestive of pneumonia enables the formulation of a therapeutic strategy superior to that based exclusively on clinical evaluation. Bronchoscopic techniques, when performed before the introduction of new antibiotics, enable physicians to identify most patients who need immediate treatment, and help select optimal therapy in a safe and well-tolerated manner. These techniques also avoid resorting to broad-spectrum coverage of all patients who develop a clinical suspicion of infection.239 The full impact of this decision tree on patient outcome remains controversial.235,236 Yet, being able to withhold antimicrobial treatment from some patients without infection may constitute a distinct advantage in the long-term: It minimizes the emergence of resistant microorganisms in the ICU and redirects the search for another (the true) infection site.
In patients with clinical evidence of severe sepsis and rapid worsening organ dysfunction, hypoperfusion, or hypotension, or patients with a very high pretest probability of disease, the initiation of antibiotic therapy should not be delayed while awaiting bronchoscopy. Patients should be given immediate antibiotics. In this situation, simple nonbronchoscopic procedures find their best justification, allowing distal pulmonary secretions to be obtained on a 24-hour basis, just before starting new antimicrobial therapy.
Despite broad experience with PSB and BAL, it remains unclear which should be used. Most investigators prefer BAL over PSB to diagnose bacterial pneumonia, because BAL (a) has a slightly higher sensitivity to identify VAP-causative microorganisms, (b) enables better selection of an empiric antimicrobial treatment before culture results are available, based on microscopically examined cytocentrifuged preparations, (c) is less dangerous for many critically ill patients, (d) is less costly, and (e) may provide useful clues for the diagnosis of other types of infections. Nevertheless, a very small return on BAL may contain only diluted material from the bronchial rather than alveolar level, and thus give rise to false-negative results, particularly in patients with very severe chronic obstructive pulmonary disease. In these patients, the value of BAL is greatly diminished and PSB is preferred.184
When bronchoscopy is not available, we recommend replacing bronchoscopy in the algorithm in Figure 46-3 by one of the simplified nonbronchoscopic diagnostic techniques, or following the strategy described by Singh et al (see Figure 46-3). Such an approach avoids prolonged treatment of patients with a low likelihood of infection, while allowing immediate treatment of patients with VAP.
Treatment
Antimicrobial therapy of patients with VAP is a two-stage process. The first stage involves administering broad-spectrum antibiotics to avoid inappropriate treatment in patients with true bacterial pneumonia.2 The second stage focuses on trying to achieve this objective without overusing and abusing antibiotics. In general, the first goal can be accomplished by identifying patients with pneumonia in a rapid fashion and starting therapy with an empirical regimen that is likely to be accurate. This requires that the choice be driven by anticipation of the likely etiologic pathogens, modified by knowledge of local patterns of antimicrobial resistance and local microbiology. The second goal involves combining a number of different steps, including stopping therapy in patients with a low probability of the disease, commitment to focus and narrow treatment once the etiologic agent is known, switching to monotherapy after day 3, and shortening duration of therapy to 7 to 8 days in most patients, as dictated by the patient’s clinical response and information about the bacteriology.
Failure to initiate prompt appropriate and adequate therapy (the etiologic organism is sensitive to the therapeutic agent, the dose is optimal, and the correct route of administration is used) has been a consistent factor associated with increased mortality.32,155–157 Because pathogens associated with inappropriate initial empiric antimicrobial therapy mostly include antibiotic-resistant microorganisms, such as P. aeruginosa, Acinetobacter spp., Klebsiella pneumoniae, Enterobacter spp., and MRSA, patients at risk for infection with these organisms should initially receive a combination of agents that can provide a very broad spectrum of coverage (Table 46-3).2,173 Several observational studies have now confirmed that the use of a regimen that combines initially a broad-spectrum β-lactam with an aminoglycoside increases the proportion of patients appropriately treated as compared to monotherapy or to a regimen combining a β-lactam with a fluoroquinolone.162,240–242 Only patients with early onset infection, mild or moderate disease severity, and no specific risk factors for multiresistant strains, such as prolonged duration of hospitalization (≥5 days), admission from a health care–related facility, recent prolonged antibiotic therapy, and specific local epidemiologic data, can be treated with a narrow-spectrum drug, such as a nonpseudomonal third-generation cephalosporin.1,2,243

Full access? Get Clinical Tree