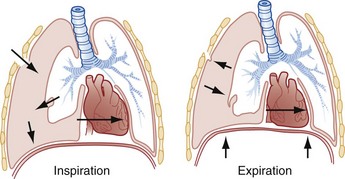
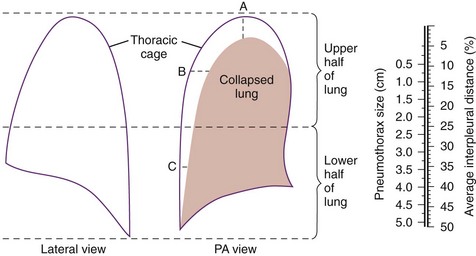
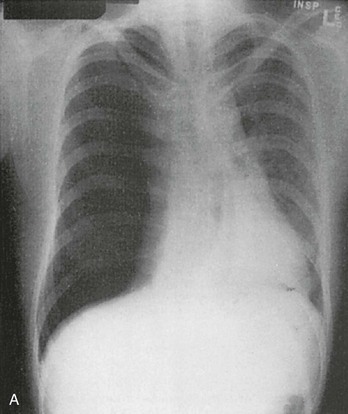
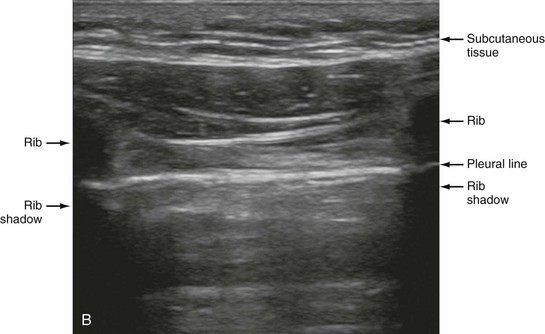
Chapter 77 Pleural disease is commonly encountered in the emergency department (ED). Presentations range in severity from asymptomatic pleural effusion to tension pneumothorax. This chapter reviews the two most common nontraumatic pleural problems: spontaneous pneumothorax and pleural inflammation with effusion. Pleural space problems associated with trauma are discussed in Chapter 45, and the approach to a patient with pleuritic chest pain in Chapter 26. The incidence of primary spontaneous pneumothorax is approximately 15 cases per 100,000 population per year among men and 5 cases per 100,000 population per year among women.1,2 Primary spontaneous pneumothorax typically occurs in healthy young men of taller than average height. Factors associated with primary spontaneous pneumothorax include cigarette smoking and changes in ambient atmospheric pressure. Familial patterns suggest an inherited propensity in some cases of primary spontaneous pneumothorax. Mitral valve prolapse and Marfan syndrome are associated with spontaneous pneumothorax in the absence of clinically apparent lung disease. Approximately one third of spontaneous pneumothoraces occur in the context of underlying pulmonary disease (Box 77-1). The incidence of secondary spontaneous pneumothorax is three times higher in men. The most common condition associated with secondary spontaneous pneumothorax is chronic obstructive pulmonary disease (COPD), which accounts for nearly 70% of cases. Patients with severe COPD (e.g., with forced expiratory volume in 1 second below 1 L) are at highest risk. The incidence of spontaneous pneumothorax among patients hospitalized for emphysema is 0.8% and for asthma 0.3%. Spontaneous pneumothorax is rare in childhood. The principles of diagnosis, imaging, treatment, and surgical management for pediatric primary spontaneous pneumothorax are similar to those for adult pneumothorax.1 In tension pneumothorax, the alveolar-pleural defect acts as a one-way valve, allowing air to pass into the pleural space during inspiration and trapping it there during expiration (Fig. 77-1). This trapping leads to progressive accumulation of intrapleural air and increasingly positive intrapleural pressure, causing compression of the contralateral lung with asphyxia and worsening hypoxia. Intrapleural pressure exceeding 15 to 20 mm Hg impairs venous return to the heart. If the situation is allowed to progress, cardiovascular collapse and death ensue. In primary spontaneous pneumothorax, disruption of the alveolar-pleural barrier occurs when a subpleural bulla (or bleb), typically located at the lung apex, ruptures into the pleural space. Subpleural bullae are found in almost all patients who undergo surgical treatment for primary spontaneous pneumothorax and are identified on computed tomography (CT) of the chest in 90% of cases. The cause of these bullae may be related to degradation of elastic fibers within the lung and an imbalance in the protease-antiprotease and oxidant-antioxidant systems.2 Although suggested by the patient’s history and physical examination, the diagnosis of pneumothorax is generally made with the chest radiograph. The classic radiographic appearance is that of a thin, visceral pleural line lying parallel to the chest wall, separated by a radiolucent band devoid of lung markings. The average width of this band can be used to estimate the size of the pneumothorax with a fair degree of accuracy (Fig. 77-2). In general, however, it is more reasonable simply to characterize the pneumothorax as small, moderate, large, or total. The estimated size of the pneumothorax and the patient’s clinical status can be useful in guiding management decisions. Tension pneumothorax is a clinical diagnosis, and delay in treatment to obtain radiographic confirmation is inadvisable. When the diagnosis of tension pneumothorax is not apparent clinically and a chest radiograph is obtained, the classic appearance is one of complete lung collapse with gross distention of the thoracic cavity on the affected side and shift of mediastinal structures across the midline (Fig. 77-3A). In patients with underlying pulmonary disease, however, pleural adhesions and lack of lung elasticity may mask the fact that a pneumothorax is under significant positive pressure. When pneumothorax is suspected but not seen on a standard chest radiograph, an expiratory film may be obtained. Theoretically, the volumes of the lungs and the chest cavity are reduced during expiration so that the relative size of the pneumothorax is enhanced. Although expiratory films are occasionally helpful in identifying a small apical pneumothorax, their routine use does not improve diagnostic yield. In critically ill patients for whom only a supine chest radiograph can be obtained, the finding of a “deep sulcus” (i.e., a deep lateral costophrenic angle) can suggest the presence of pneumothorax on that side (see Fig. 77-3A). Chest CT is considered the gold standard for the diagnosis of pneumothorax; however, it requires that patients be stable enough for transport. Although portable chest radiography is convenient and commonly used, it has poor overall sensitivity.3 Point-of-care thoracic ultrasound is a rapid and accurate diagnostic aid performed and interpreted by the clinician at the bedside.4,5 Bedside thoracic ultrasound is more sensitive than a supine chest radiograph for the diagnosis of pneumothorax and avoids ionizing radiation.6,7 In addition to routine use for the diagnosis of both traumatic and spontaneous pneumothorax, thoracic ultrasound can be used to evaluate for postprocedural pneumothorax and for the diagnosis of occult pneumothorax in critically ill patients.8,9 In normal lung the closely opposed visceral and parietal pleura create the appearance of shimmering or sliding of the pleural interface during respiration. Assessment for pneumothorax is optimally performed with a high-frequency linear probe to most accurately visualize the lung sliding at the pleural line (see Fig. 77-3B). In supine patients, air rises; scanning a few interspaces on the anterior chest wall along the midclavicular line can effectively evaluate the most likely areas for pneumothorax. Extending the examination to the lateral chest wall can help identify the “lung point” or boundary between normal lung and pneumothorax and is highly specific for the detection of pneumothorax.10 Visualization of lung sliding at the pleural line effectively rules out pneumothorax in the area being scanned. A small pneumothorax may not be detected with ultrasound, and false-positive results can occur in patients with blebs, pleural scarring, or severe acute respiratory distress syndrome (ARDS) or in single-lung intubation. Although there is no universal agreement on the optimal treatment of patients with a first episode of primary spontaneous pneumothorax, data suggest that aspiration may be equally effective as chest tube drainage and clearly causes much less patient discomfort.11 Advantages of simple aspiration include low morbidity, lack of invasiveness, and overall cost savings, with reported rates of successful outcome ranging from 45 to 71%.12 Success is less likely when the patient is older than 50 years or the volume of air aspirated exceeds 2.5 L, suggesting a continuing air leak. If aspiration fails to reexpand the lung fully, the catheter can be attached to a water-seal device or to a one-way Heimlich valve and managed like a small-caliber chest tube. Most recurrent spontaneous pneumothoraces should be managed with tube thoracostomy because less invasive approaches (i.e., observation or simple aspiration) are associated with significantly lower success rates.13 Similarly, patients who have respiratory distress, have tension physiology, or are likely to require mechanical ventilation should undergo tube thoracostomy to reexpand the lung definitively. Also, if there is significant pleural fluid (hemothorax or hydrothorax), tube thoracostomy is required. Finally, tube thoracostomy may be considered in uncomplicated cases of primary spontaneous pneumothorax either as a first-line intervention or after a less invasive approach (i.e., observation or simple aspiration) fails.
Pleural Disease
Spontaneous Pneumothorax
Pathophysiologic Principles
Clinical Features
Management

Full access? Get Clinical Tree