Physiologic Response to Injury
Luke P.H. Leenen
I.
Multiple changes occur in an organism following injury. The initial responses are protective, followed by compensation for the changes experienced and attempts to restore integrity and homeostasis. After the initial physiologic mechanisms to compensate, processes are initiated to prepare the organism to repair and ultimately heal. The response to injury is composed of a local response, tissue level reaction, and a general, systemic, response. After the first hit and the initial response, an individually determined response is evoked by the organism on a systemic level, depending on the extent of the injury.
Immediate changes experienced after injury (Table 2-1):
Tissue injury, disrupting the integrity of the tissues
Hemorrhage, followed by:
Hypovolemia
Hypercarbia
Hypoxia
Acidosis
Coagulopathy
Tissue edema and volume shifts
The immediate changes are followed by a fight or flight response, which is an adrenocortical response to stress and leads to increased production of corticosteroids and catecholamines. This redistributes the blood flow to essential organs such as the heart and brain. The hypothalamic–pituitary (ACTH, endorphins, ADH)–adrenal (corticosteroids, epinephrine) axis is activated, as well as kidney response (renin) and sympathetic response (catecholamines). These activations lead to a hormonally driven homeostatic response, composed of:
Increase in heart rate
Increase in respiratory rate
Fever
Leukocytosis
This immediate response is supported by:
Excitation of the sympathetic nervous system, because of the pain elicited by the trauma. This leads to hypertension, a high pulse rate and cortisol release.
Stimulation of baroreceptors, atrial as well arterial, leads to vasoconstriction and increased hormonal activity.
Specific changes in the blood composition lead to stimulation of chemoreceptors, which elicit compensatory changes.
After the immediate response, several other processes ensue:
Hemostasis
Volume shifts
Local and systemic immune response
Initiation of tissue repair through local tissue reaction involving (Fig. 2-1):
Complement. Based on ischemia and endothelial injury; invading microorganisms also may activate plasma proteins
Oxygen radicals
Produced by leukocytes and parenchymal cells
Consist of toxic products, such as hydrogen peroxide, superoxide, and hydroxyl radicals
Table 2-1 Organ System Response
Organ system
Physiologic response
Cardiovascular
Tachycardia
Blood pressure
Cardiac output higher
Renal
Lower output
Adrenal
Higher cortisol release
Pulmonary
Increased minute volume:
By higher frequency and tidal volume
CNS
Altered mental state (direct or indirect)
Splanchnic
Decreased blood flow
Disintegrated barrier function
General
Edema:
- Higher body salt
- Interstitial fluid
- Capillary permeability
- Hyponatremia
- Local cytokines
Leukocytosis/platelets- Higher body salt
Cytokines. Responses by local tissues, but also by systemic organs such as the liver
Eicosanoids. Prostaglandins, leukotrienes, and thromboxanes
Nitrogen oxide. From endothelial cells as a homeostatic factor for the immediate regulation of the blood pressure. Increased by inflammation and may contribute to profound hypotension and decompensated hemorrhagic shock.
Others. Release of several growth factors such as platelet-derived growth factor and endothelial-derived growth factor
II. Hemostasis: The Effect of Trauma on Coagulation
Coagulation is a complex process which attempts to maintain integrity of the vascular system. Important in this respect, is the process of thrombocyte aggregation to plug the gap with thrombus formation. Coagulation abnormalities have long been thought to be only secondary to hemodilution from resuscitation and hypothermia. We now understand that tissue injury directly influences coagulation (acute traumatic coagulopathy). As mentioned above, the coagulation system is influenced by our treatment, as following factors influence coagulation:
Dilution
Hypothermia
Hypoxia
Acidosis
Currently, however, trauma and, more importantly, tissue hypoxia are seen as important, independent factors in the development of coagulation disorders after trauma.
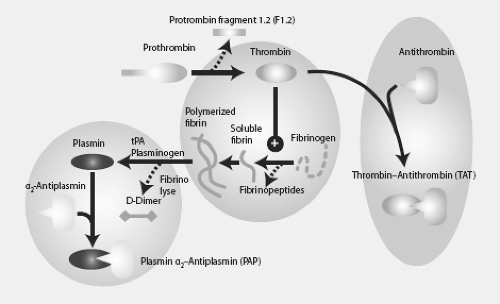
Trauma-induced coagulopathy (TIC) and its component acute traumatic coagulopathy lead to endogenous coagulopathy, which is present in up to 25% of trauma patients entering the emergency department. It is induced by a combination of tissue injury and shock and related to tissue hypoperfusion. The driver seems to be the systemic activation of the protein C pathway (Fig. 2-2), resulting in:
Coagulation inhibition. Thrombomodulin is presented by the damaged endothelium and forms the Thrombomodulin complex which can no longer cleave fibrinogen
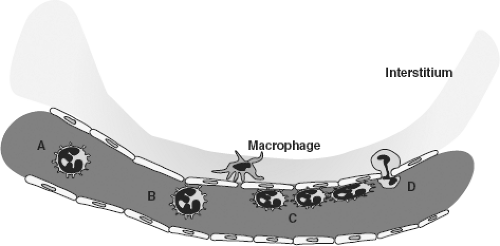
Figure 2-1. Cascade of processes after injury (left lower corner). Role of various cells and cytokines and their actions. Redrawn after Hietbrink et al. WJES 1:15.
Decreased fibrinogen utilization
Increased fibrinolysis. Tissue plasminogen activator (tPA) is presented by the endothelium by injury and hypoperfusion. tPA cleaves plasminogen to initiate fibrinolysis. Moreover, a potent inhibitor of tPA, plasminogen activator inhibitor-1 (PAI-1) is consumed leading to hyperfibrinolysis.
Elevated admission INR is an independent predictor of poor outcome in trauma patients. Currently, thromboelastography (TEG) is utilized to further clarify the coagulopathy of trauma.
III. Continued Volume Shifts
Hypoxemia at the tissue level and the effects of microparticles released after tissue damage compromise the integrity of the endothelial lining and the natural barrier is lost, resulting in a rapid redistribution of the fluids over the compartments (Fig. 2-3).
Three phases of volume shifts after trauma:
Phase I: Shock and active hemorrhage
This phase controls bleeding and lasts from admission to the end of operation for hemostasis. Pre-capillary vasoconstriction reduces the hydrostatic efflux of fluid, electrolytes, and protein into the interstitium. The interstitial matrix and plasma volume are contracted as electrolytes and proteins are mobilized into the circulation.
Phase II: Obligatory extravascular fluid sequestration
To restitute fluid in the interstitium, volume shifts into the interstitial and intercellular spaces, leading to oliguria, if insufficient fluid is given. An obligatory sequestration phase follows. This sequestration gives a modest rise in the volume of the intercellular fluid compartment and a marked expansion of the interstitial space. This leads to a reduction in plasma volume and plasma proteins and albumin relocation into the interstitial space.
Phase III: Fluid mobilization and diuresis
In this phase, fluid again is mobilized from the interstitial spaces, resulting in an acute increase in plasma volume sometimes resulting in volume overload, hypertension, and pulmonary dysfunction.
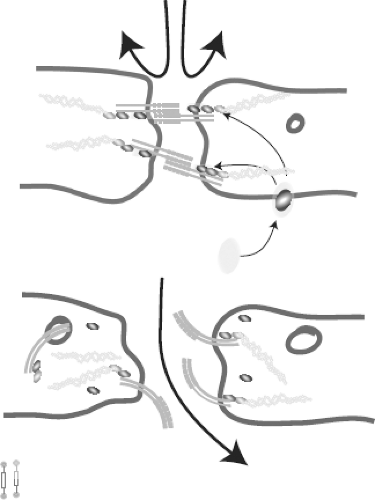 Figure 2-3. The integrity of the endothelial lining is compromised resulting in loss of barrier function and subsequent fluid loss to the interstitium. Redrawn after Le and Slutsky. NEJM 2010;363:689.

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|