Chapter 7 Physiologic and Pathophysiologic Responses to Intubation
I Background
Laryngoscopy, endotracheal intubation, and other airway manipulations (e.g., placement of a nasopharyngeal or oropharyngeal supralaryngeal airway) are noxious stimuli that may induce profound changes in cardiovascular physiology, primarily through reflex responses. Although these responses may be of short duration and of little consequence in healthy individuals, serious complications can occur in patients with underlying coronary artery disease,1,2 reactive airways,3,4 or intracranial neuropathology.5,6
II Cardiovascular Responses during Airway Manipulation
A Cardiovascular Reflexes
The cardiovascular responses to noxious airway manipulation are initiated by proprioceptors responding to tissue irritation in the supraglottic region and in the trachea.7 Located in close proximity to the airway mucosa, these proprioceptors consist of mechanoreceptors with small-diameter myelinated fibers, slowly-adapting stretch receptors with large-diameter myelinated fibers, and polymodal endings of nonmyelinated nerve fibers.8 (The superficial location of these proprioceptors and their nerves explains why topical local anesthesia of the airway is such an effective means of blunting cardiovascular responses to airway interventions.) The glossopharyngeal and vagal afferent nerves transmit these impulses to the brainstem, which, in turn, causes widespread autonomic activation through the sympathetic and parasympathetic nervous systems. Bradycardia, often elicited in infants and small children during laryngoscopy or intubation, is the autonomic equivalent of the laryngospasm response. Although seen only rarely in adults, this reflex results from an increase in vagal tone at the sinoatrial node and is virtually a monosynaptic response to a noxious stimulus in the airway.
In adults and adolescents, the more common response to airway manipulation is hypertension (HTN) and tachycardia mediated by the cardioaccelerator nerves and sympathetic chain ganglia. This response includes widespread release of norepinephrine from adrenergic nerve terminals and secretion of epinephrine from the adrenal medulla.9 Some of the hypertensive response to endotracheal intubation also results from activation of the renin-angiotensin system, including release of renin from the renal juxtaglomerular apparatus, which is innervated by β-adrenergic nerve terminals.
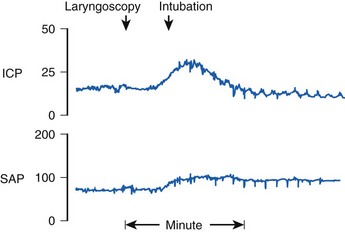
In addition to activation of the autonomic nervous system, laryngoscopy and endotracheal intubation result in stimulation of the central nervous system, as evidenced by increases in electroencephalographic (EEG) activity, cerebral metabolic rate, and cerebral blood flow (CBF).10 In patients with compromised intracranial compliance, the increase in CBF may result in elevated intracranial pressure (ICP), which, in turn, may result in herniation of brain contents and severe neurologic compromise.
B Intubation in the Presence of Cardiovascular Disease
It follows, then, that neuroendocrine responses to airway manipulation resulting in tachycardia and HTN may result in a variety of complications in patients with cardiac disease, myocardial ischemia chief among them. This set of circumstances is responsible for episodes of ischemic electrocardiographic ST-segment depression and increased pulmonary artery diastolic blood pressure (BP) that may be seen when intubation is performed in patients with arteriosclerosis; occasionally, these episodes presage the occurrence of a perioperative myocardial infarction.2 However, short ischemic episodes (<10 minutes) evidenced by electrocardiographic ST-segment depression, such as those that may be experienced only during airway manipulation, have not been shown to correlate with postoperative myocardial infarction. In contrast, ST-segment changes of a single duration lasting longer than 20 minutes (mean SD 20 ± 30 minutes) or cumulative durations of longer than 1 hour (mean SD 1 ± 2 hours) do seem to be an important factor associated with adverse perioperative cardiac outcomes.11,12
C Implications for Patients with Neurovascular Disease
Intracranial aneurysms and arteriovenous malformations (AVMs) often arise with a small “sentinel” hemorrhage that serves as a warning of worse things to come. During subsequent periods of elevated arterial BP, these lesions are likely to rebleed, resulting in sudden and permanent neurologic injury. Many neurosurgeons and interventional neuroradiologists attempt to stabilize cerebral aneurysms and AVMs soon after hospitalization in an effort to minimize the risk of rebleeding. This means that the patient presents for anesthesia at a time when the clot tamponading the aneurysm or AVM is particularly delicate, and a small increase in arterial transmural pressure could cause rerupture. One of the times at which this is most likely to occur is when the arterial BP and the HR are increased in response to endotracheal intubation.5 Therefore, neurosurgical anesthesiologists pay meticulous attention to attenuating these responses during the course of anesthetic induction and endotracheal intubation.
D Intubation in Patients with Neuropathologic Disorders
Reflex responses to endotracheal intubation are also a potential hazard to patients with compromised intracranial compliance resulting from neuropathologic processes such as intracranial mass lesions, brain edema, or acute hydrocephalus. Uncontrolled coughing can result in a marked increase in intrathoracic and intra-abdominal pressure that, in turn, increases cerebrospinal fluid pressure and may result in impairment of cerebral perfusion. In patients with impaired cerebral autoregulation (e.g., brain trauma, cerebrovascular accidents, neoplasms), the normal tendency for CBF to remain constant over the mean BP range of 50 to 150 mm Hg is impaired. When endotracheal intubation causes an increase in arterial BP, there is a marked increase in CBF and cerebral blood volume, which in turn can cause dangerous increases in ICP.6 This effect is magnified by the fact that noxious stimuli, such as airway manipulation, result in increased CBF, which summates with the hypertensive BP response, occasionally causing profound increases in ICP (Fig. 7-1).
E Neuromuscular Blocking Drugs and Cardiovascular Responses
Neuromuscular blocking drugs (NMB) are often administered to optimize conditions for intubation. Accordingly, it is appropriate to consider the cardiovascular and cerebrovascular responses to the administration of these agents. Indeed, the hypertensive-tachycardic response to endotracheal intubation was not identified until NMB agents were introduced into clinical practice, because before that time intubation was performed only with the patient under such deep levels of anesthesia that relatively little cardiovascular response was generated.13
The depressor effects of benzylisoquinolinium relaxants (atracurium and mivacurium) are mediated by histamine release.14 This effect could be viewed as a potential antagonist to the pressor response to laryngoscopy and endotracheal intubation. In the case of patients at risk for intracranial HTN, however, histamine-induced cerebral vasodilation may produce increases in ICP even as the BP falls.15 By contrast, pancuronium, rocuronium, and, to a lesser extent, vecuronium may initiate a hyperdynamic cardiovascular state that can potentiate the cardiovascular responses seen after endotracheal intubation in lightly anesthetized patients. The faster onset of rocuronium (doses of up to 2 mg/kg have a 90% chance of providing perfect intubating conditions) are the reason for its current widespread use as an alternative to succinylcholine for rapid-sequence intubation (RSI) and in operations expected to last longer than 1 hour.16
Succinylcholine, or diacetylcholine, is associated with bradycardia in children, particularly when doses are repeated, but is a cardiovascular stimulant in adults. This phenomenon is often associated with activation of the EEG, and patients with brain tumors may sustain marked increases in ICP after succinylcholine administration if intracranial compliance is compromised and cerebrovascular autoregulation is impaired.17 This has been shown in dogs to be a result of increased CBF related primarily to succinylcholine-induced increases in afferent muscle spindle activity at the time of fasciculation and secondarily to an elevated arterial carbon dioxide tension from fasciculation-induced carbon dioxide production.18 The evidence to substantiate the clinical relevance of these findings is lacking, however. Whereas it has been reported that succinylcholine administered to patients with brain tumors may elevate ICP by a mean of 5 to 12 torr, cerebral perfusion pressure does not change significantly, and a negative effect on neurologic outcome has not been documented.19,20 Additionally, this phenomenon can be prevented by pretreatment with defasciculating doses of nondepolarizing NMB drugs.19 Further, when adequate ventilation is maintained, succinylcholine administered to intubated patients being treated for intracranial HTN of various causes or to those who have suffered severe head injuries caused by blunt trauma had no effect on ICP, cerebral perfusion pressure, or CBF.21,22 As a result, succinylcholine is still considered a first-line agent for RSI in patients with acute head injury but is ideally used after pretreatment with a nondepolarizing agent and in the presence of slight hypocapnia.
F Cardiopulmonary Consequences of Positive-Pressure Ventilation
It should also be noted that both hypoxemia and hypercapnia lead to a stress-induced catecholamine response, which may mask other potential causes of hypotension. This becomes readily apparent only after intubation in critically ill patients. Prophylactic volume expansion and the immediate availability of vasoactive infusions decrease severe hemodynamic collapse in this situation.23
III Prevention of Cardiovascular Responses
A Technical Considerations: Minimizing Stimulation of Airway Proprioceptors
As a general rule, cardiovascular responses to airway maneuvers can be minimized by limiting airway proprioceptor stimulation, starting with manipulation of the larynx itself. For instance, cricoid cartilage pressure with a posterior force of 4.5 kg is widely used to prevent regurgitation of gastric contents or to facilitate laryngeal visualization. In a double-blind study, cricoid pressure resulted in a significantly greater HR and BP response to endotracheal intubation than occurred in patients whose cricoid area was gently palpated.24 This is a little-recognized effect of cricoid pressure that should be considered when estimating the risk-benefit ratio of this procedure in individual patients.
Laryngoscopy itself is a moderately stimulating procedure, and use of a straight blade (Miller blade) with elevation of the vagally innervated posterior aspect of the epiglottis results in significantly higher arterial BP than does use of a curved blade (Macintosh or Corazzelli–London–McCoy [CLM]).25 Newer video and optical laryngoscopes, which do not require alignment of the laryngeal axes for adequate visualization of the vocal cord inlet and subsequent intubation, have the potential to minimize the pressor response to airway manipulation by reducing the amount of force needed to displace oropharyngeal tissues and limiting cervical spine motion compared to traditional laryngoscopy with a Macintosh laryngoscope blade.26 Nonetheless, reports documenting this advantage are few.
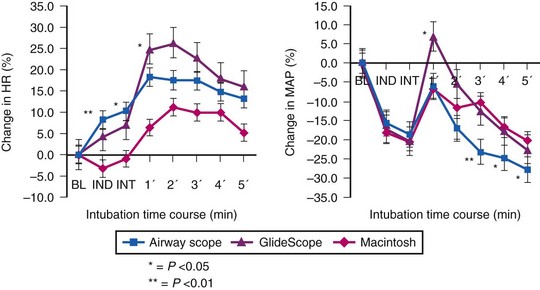
Use of the Pentax-AWS video laryngoscope (Pentax, Tokyo, Japan) has been reported to attenuate the hemodynamic response to endotracheal intubation after fentanyl/propofol induction when compared to either the GlideScope (Verathon, Bothell, WA) or the Macintosh laryngoscope (Fig. 7-2).27 This finding is not universal. An earlier study comparing the Pentax-AWS to Macintosh laryngoscopy reported no significant differences in systolic BP, diastolic BP, or HR after intubation, and a separate study comparing the GlideScope and Macintosh laryngoscopy also failed to find significant differences in hemodynamic values at any point in the study.28 None of these studies included patients with known cardiac disease or chronic HTN, who often have exaggerated pressor responses to stimulation; the newer airway devices may have greater value among this group compared with traditional laryngoscopy.
The act of passing an endotracheal tube (ETT) is far more hemodynamically stimulating than just laryngoscopy. Surprisingly, the use of a lighted intubation stylet fails to prevent hemodynamic stimulation when the ETT is advanced past the vocal cords.29 Insertion of a conventional laryngeal mask airway (LMA) after induction of general anesthesia with thiopental or propofol and fentanyl has been shown to cause less cardiovascular and endocrine response than laryngoscopy or endotracheal intubation.30–33 The LMA has the advantage of avoiding the vagally mediated infraglottic stimulation entailed by the use of a laryngoscope, thus enabling lighter levels of general anesthesia. Furthermore, because muscle relaxation is not required for airway control, spontaneously initiated ventilation is possible, with avoidance of the adverse hemodynamic consequences of PPV. In contrast, endotracheal intubation using the intubating LMA (iLMA) resulted in a hemodynamic and endocrine response similar to that resulting from direct laryngoscopy and intubation after propofol induction.34 Therefore, if endotracheal intubation is necessary, there does not appear to be a hemodynamic advantage to instrumenting the airway with the iLMA.
Whatever the technique employed to manage the airway, it must be emphasized that the hypertensive-tachycardic response to intubation is a manifestation of insufficient anesthesia. Insofar as the pressor response can also be influenced by prolonged intubation time, rapid first-attempt success is also of particular importance.7
B Topical and Regional Anesthesia
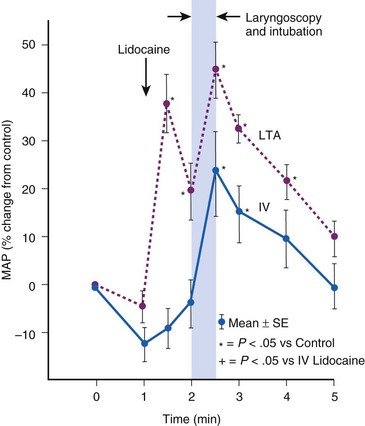
Topical anesthesia applied to the upper airway is effective in blunting hemodynamic responses to endotracheal intubation,35,36 but it has almost invariably proved to be less effective than systemic administration of lidocaine. During general anesthesia, rigid laryngoscopy and instillation of lidocaine solution initiate the same adverse reflexes caused by placement of an ETT (Fig. 7-3).37 Furthermore, a laryngotracheal spray of lidocaine solution may, in itself, produce profound cardiovascular stimulation in adults, and in children it may produce the same sort of bradycardic response associated with endotracheal intubation.38 If topical lidocaine is administered to the upper airway, there should be an intervening period of at least 2 minutes to allow initiation of anesthetic effect before airway instrumentation begins.39
Excellent topical anesthesia of the airway obtained before awake flexible fiberoptic intubation was responsible for reports suggesting that there was less cardiovascular stimulation after this procedure than after intubation with a rigid laryngoscope.40 Later studies performed with patients under general anesthesia demonstrated no difference between the two modes of intubation with regard to hemodynamic impact, probably because the more profound stimulus results from placement of the ETT below the level of the glottis.41–44
Increasing the concentration of lidocaine used, and thus the total dose, also does not appear to mitigate this effect, although it may improve intubating conditions during awake flexible fiberoptic intubation.45,46 Although both 2% and 4% lidocaine administered through an epidural catheter in the working channel of the flexible fiberoptic bronchoscope by a “spray-as-you-go” technique provided similar intubating conditions and hemodynamic profiles, the former resulted in a smaller overall dose, lower plasma levels, and therefore less chance for toxicity reactions.46 Lower concentrations of lidocaine (1%) provided lower plasma levels and similar hemodynamics but appeared to provide less optimal intubating conditions than atomized 2% lidocaine when used for topical anesthesia before airway manipulation.45
In contrast to topical anesthesia of the airway, which appears to provide inconsistent benefit, regional nerve blocks involving the sensory pathways from the airway prevent hemodynamic responses to intubation. The superior laryngeal nerve (SLN) innervates the superior surface of the larynx, and the glossopharyngeal nerve innervates the oropharynx. Depositing local anesthetic on each cornu of the hyoid bone can block the SLN. Blockade of the glossopharyngeal nerve at the tonsillar pillars (sensory distribution above the level of the epiglottis) potentiates this effect by decreasing the stimulus of laryngoscopy.47 The inferior surfaces of the larynx and trachea require topical anesthesia, however, because they are innervated by the recurrent laryngeal nerve and the vagus, which cannot be directly blocked. With the preceding combination, awake patients exhibit little response as the ETT is inserted.
Instillation of lidocaine via an ETT to prevent alterations in cerebrovascular hemodynamics in patients with severe head injury may be of some benefit. A dose of 1.7 mg/kg lidocaine instilled at body temperature given slowly (1 mL/sec) through a fine tube advanced to the end of the ETT but not in contact with the tracheal mucosa was reported to be efficacious in half of the patients treated.48
C Inhalational Anesthetics
For inhalational anesthetics, endotracheal intubation using doses in the range of the minimum alveolar concentration (1 MAC) resulted in marked cardiovascular stimulation during anesthesia with nitrous oxide (N2O) supplemented with either halothane or morphine.49 It should not be surprising that 1 MAC is insufficient, because it is known that approximately 1.5 to 1.6 MAC is needed to block the adrenergic and cardiovascular responses to a simple surgical skin incision (MAC-BAR).50 The dose of anesthetic required to prevent coughing during endotracheal intubation with sevoflurane may exceed MAC by a factor of 2.86 in adults,51 although this factor appears to be close to 1.3 in children.52
Accordingly, it appears that the dose of volatile anesthetic required to block the cardiovascular response to endotracheal intubation must be inordinately high, resulting in profound cardiovascular depression before endotracheal intubation.53 From a cerebrovascular viewpoint, this approach is totally impractical, because high doses of volatile anesthetics cause cerebral vasodilation and marked increases in ICP in patients with compromised intracranial compliance. Furthermore, from a cardiovascular point of view, the arterial hypotension and reduced cerebral perfusion pressure before intubation would be entirely unacceptable for patients with cerebrovascular disease or brain injury.
D Intravenous Agents
Propofol, barbiturates, and benzodiazepines are all associated with profound hypotension at doses that suppress the hemodynamic and ICP responses to intubation.54–56 In the case of etomidate, the effective dose for blocking the cardiovascular response to intubation can be identified by a burst-suppression pattern on the cortical surface EEG, indicating fairly deep cerebral depression.57 Because etomidate supports BP at such deep levels of anesthesia, it is probably the only contemporary agent that, by itself, can achieve suppression of cardiovascular responses without first producing undue arterial hypotension and compromise of coronary and cerebral perfusion.
Opioids are the adjuvants most commonly administered in addition to other IV or inhaled agents to facilitate induction of anesthesia and subsequent airway manipulation. Their use in this capacity relates to their historical use as part of a N2O-narcotic anesthetic often used in patients with marginal cardiac reserve. For example, Bennet and Stanley compared the cardiovascular responses after administration of N2O-morphine 0.4 mg/kg versus N2O-fentanyl 4 µg/kg 10 minutes before intubation. The HR, cardiac output, and systolic and mean BP were reduced compared to baseline and remained unaffected by intubation in the N2O-fentanyl group, but these parameters were all significantly elevated compared with preanesthetic controls in the N2O–morphine group.58 Whereas the assumed potency of fentanyl in this study was 100 times that of morphine, the lack of effect of morphine suggests that, with respect to suppression of pressor responses to laryngotracheal manipulation, fentanyl is more than 100 times as potent.
As reported by Bennett and Stanley58 and later by other investigators,59 fentanyl may not achieve its peak central nervous system effect until 10 minutes after bolus IV injection. Fentanyl appears to provide blunting of hemodynamic responses in a graded manner: 2 µg/kg IV given several minutes before induction only partially prevented HTN and tachycardia during an RSI with thiopental and succinylcholine. In this situation, 6 µg/kg was considerably more effective.60 Chen and coworkers reported almost complete suppression of hemodynamic response to intubation with both 11 and 15 µg/kg of IV fentanyl, whereas higher IV doses (30 to 75 µg/kg) allowed only a very occasional response to intubation.61
In doses that prevent hemodynamic response to intubation, however, fentanyl is not a short-acting agent, and the risk of prolonged postoperative respiratory depression must be weighed against the advantages of perioperative cardiovascular stability. With this risk in mind, it has been observed that pretreatment with 2 µg/kg IV fentanyl given 10 minutes before intubation during an infusion of propofol sufficient to reduce the Bispectral Index Score to 45 prevented a significant increase in HR or BP compared with awake preanesthetic values.10 Similar results were observed when intubation was performed after administration of fentanyl, 2 µg/kg, and propofol bolus doses of 2.0 to 3.5 mg/kg.10
Fentanyl and propofol require 6.4 and 2.9 minutes, respectively, to achieve effect-site equilibrium after IV bolus administration.10 Therefore, the common practice of administering 1 to 2 mL (50 to 100 µg) just before or almost simultaneously with other induction medications would not be expected to have any effect based on inadequate dose and inappropriate timing of administration. Rather, this may provide a more plausible explanation for hypotension during the minutes-long quiescent period between endotracheal intubation and actual surgical incision. It is strongly recommended that laryngoscopy and intubation be timed to coincide with the peak effect of these agents.
Opioids with shorter onset and offset times have some advantages over fentanyl for modulating circulatory responses to intubation. Alfentanil has a smaller steady-state distribution volume and shorter terminal elimination half-life than fentanyl.62 Ausems and colleagues demonstrated that an alfentanil plasma concentration of 600 ng/mL effectively prevented hemodynamic responses to intubation during induction of N2O anesthesia.63 This was achieved by a 30-second infusion of alfentanil at 150 µg/kg. During this induction period, N2O and succinylcholine were also administered. Only 5 of the 35 patients studied sustained an increase in HR or BP greater than 15% above preinduction values.
Remifentanil has been found to be highly effective in preventing hemodynamic responses to intubation, albeit always with the cost of impressive bradycardia or hypotension, or both, before and after airway manipulation.64 Many studies have used vagolytic agents to avoid bradycardia, at the risk of an elevated HR response after intubation. Remifentanil’s half-time for equilibration between blood and effect site is 1.3 minutes,65 and it has a brief half-life of 3 to 5 minutes due to hydrolysis by tissue and blood esterases.66 Typical remifentanil infusion rates used for blunting hemodynamic responses are 0.25 to 1.0 µg/kg/min in association with cautious propofol administration and nondepolarizing neuromuscular blockade.67 For RSI with thiopental and succinylcholine, the optimal dose of remifentanil appears to be 1.0 µg/kg administered over 30 seconds, with laryngoscopy performed 1 minute after induction. A bolus dose of 1.25 µg/kg was associated with unsatisfactory bradycardia, whereas 0.5 µg/kg resulted in excessive cardiovascular stimulation.68 This dosing recommendation is supported by another report that found remifentanil 1 µg/kg given over 30 seconds, followed by thiopental 5 mg/kg and rocuronium 1 mg/kg 100 seconds later, was more effective than lidocaine and esmolol in attenuating the hemodynamic response to RSI.69
IV lidocaine may also blunt hemodynamic and cerebrovascular responses to intubation. When given in a bolus of 1.5 mg/kg IV, it adds approximately 0.3 MAC of anesthetic potency.70 Significant reductions in hemodynamic response to endotracheal intubation have been noted when lidocaine (3 mg/kg) was used as an adjunct to high-dose fentanyl anesthesia,71 as well as during other light anesthetic techniques, such as thiopental-N2O-O2.72 However, smaller doses of lidocaine (1.5 mg/kg) have not been consistently reported to be effective in reducing the hemodynamic response to laryngoscopy and endotracheal intubation.73,74
The general anesthetic properties of lidocaine tend to reduce cerebral metabolic rate for O2 and CBF, thus lowering ICP in patients with compromised intracranial compliance.75 Theoretically, these properties of lidocaine might be exploited to mitigate rises in ICP during airway manipulation in those patients with acute intracranial pathology or compromised intracranial compliance. However, only a single human study has been reported specifically evaluating the ability of IV lidocaine to blunt intubation-related elevations in ICP. Bedford and colleagues compared 1.5 mg/kg IV lidocaine with placebo in 20 patients diagnosed with brain tumor. When administered 2 minutes before intubation, lidocaine failed to prevent a rise in ICP from the preanesthesia baseline, although the increase was more modest than that observed with the placebo (−12.1 mm Hg; 95% confidence interval, −22.8 to −1.4 mm Hg; P = 0.03).76 This dearth of direct benefit was underscored by a systematic review that also failed to identify any evidence that pretreatment with IV lidocaine before RSI consistently reduced ICP or positively affected neurologic outcome.77 This review is now more than a decade old, but because no new direct evidence has been published in the interim, its conclusion remains valid.
E Nonanesthetic Adjuvant Agents
A final means for modifying the cardiovascular responses to endotracheal intubation is prophylactic administration of vasoactive substances that directly affect the cardiovascular system. This approach was introduced in 1960 by DeVault and associates, who found that pretreatment with phentolamine, 5 mg IV, prevented the hypertensive-tachycardic response to endotracheal intubation during a light barbiturate-succinylcholine anesthetic technique.78 Since then, a large number of articles have appeared advocating the use of various vasodilators and adrenergic blocking agents as pretreatment before endotracheal intubation, including diltiazem, verapamil, and nicardipine79–82; hydralazine83; nitroprusside84; nitroglycerin85; labetalol86; esmolol80,87–89; and clonidine.90,91 Virtually all of these agents appear to be somewhat effective when compared to placebo, particularly when used in high doses.
Esmolol is the best studied of the group. In a large, multicenter, placebo-controlled trial, esmolol at doses of 100 or 200 mg suppressed the hemodynamic response to endotracheal intubation, particularly when combined with a moderate-dose opiate.87 However, esmolol doses of 200 mg were associated with a doubling of the incidence of hypotension compared to placebo. In another study, smaller doses of esmolol (1 mg/kg) had no effect on the hemodynamic response to laryngoscopy and intubation compared to placebo.80 Most recently, the combination of lidocaine (1.5 mg/kg) and esmolol at a dose of 1 mg/kg effectively attenuated the pressor response to intubation but was not as effective as 1 µg/kg remifentanil.69 Currently, the optimal use of any of these agents is undefined, although their use as adjuncts to RSI is reasonable taking into account evidence-based dosing recommendations for the situation.