105 Peritonitis and Intraabdominal Infection
 Pathogenesis
Pathogenesis
Host Defenses
The peritoneal cavity is a complex space lined with mesothelial cells in visceral and parietal layers. The healthy peritoneal cavity is quiescent immunologically but responds rapidly to bacterial contamination. Normally, about 50 to 100 mL of peritoneal fluid circulates freely among several potential and actual spaces within the peritoneal cavity.1 Net fluid movement is cephalad toward the diaphragm, facilitated by normal peristalsis, normal diaphragmatic excursions, splanchnic blood flow, and factors that maintain normal membrane permeability of the microcirculation. Conversely, ileus, mechanical ventilation, splanchnic hypoperfusion, and intraperitoneal inflammation can disrupt normal fluid movement and cause intraperitoneal fluid sequestration.
The three major intraperitoneal host defense mechanisms include clearance of bacteria by lymphatics, phagocytosis of bacteria by immune cells, and mechanical sequestration with abscess formation. A few phagocytic cells circulate as peritoneal macrophages, and opsonic proteins that facilitate phagocytosis are present. Experimentally, a small bacterial inoculum placed in the peritoneal cavity is cleared within a few minutes when the peritoneal fluid is absorbed by specialized lacunae on the undersurface of the diaphragm.1 The bacteria then pass into the central venous system via diaphragmatic and mediastinal lymphatics for disposition by systemic host defenses. When an infectious inoculum is introduced, a brisk inflammatory response attempts to localize the infection, leading to abscess formation rather than generalized peritonitis. Intraabdominal abscess formation is a source-containment process; mortality is lower for patients with abscess(es) than for patients with generalized peritonitis.2,3
With inflammatory injury, peritoneal mesothelial cells are denuded, exposing the underlying basement membrane. When platelets and fibrin come into contact with the basement membrane, fibrin polymerization occurs and produces a typical exudative rind on peritoneal surfaces. Fibrin and apoptotic neutrophils contribute to the formation of adhesions and the walls of abscesses. Normally the process is self-limited by up-regulation and/or activation of fibrinolytic factors such as plasminogen within the first week after mesothelial injury. If the insult is self-limited, peritoneal repair occurs within 3 to 5 days. Under local hypoxic conditions, the adhesions are invaded by fibroblasts, angiogenesis is up-regulated, and the adhesions become tenacious.4
Microbiology
Gastrointestinal perforation releases bacteria into the peritoneal cavity. Bacterial density within the gut lumen increases along the length of the gastrointestinal tract from the stomach to the colon. The bacteria must proliferate to cause infection, while local host defenses seek to prevent or contain the establishment of infection. In addition to the microbes present in peritoneal fluid, microbial colonization of peritoneal surfaces occurs rapidly after perforation or penetrating injury as a result of expression by the microorganisms of specific adherence factors. Enterobacteriaceae predominate within the first 4 hours but are superseded within 8 hours by members of the B. fragilis group. Adherent bacteria are difficult to eradicate by operative peritoneal lavage.5
Besides adherence factors, bacteria possess several other features that can enhance their virulence. Peptidoglycans and lipoteichoic acid in the cell walls of gram-positive bacteria, especially streptococci and staphylococci, stimulate a proinflammatory response. These organisms can elaborate exotoxins and proteases that cause tissue injury and promote the dissemination of the bacteria. Lipopolysaccharide (LPS) in the outer cell wall of gram-negative bacteria can interact with many cell types to stimulate an inflammatory response. As bacteria proliferate and the size of the inoculum increases, acidic bacterial metabolites can impair neutrophil function.6 Larger inocula can render antibiotics, particularly β-lactams, ineffective via a process called the inoculum effect.7 Additionally, bacteria demonstrate a quorum-sensing effect that maximizes survival and reproduction by altering their behavior based upon signaling pathways.8
Synergistic interactions, usually among members of the B. fragilis group and either facultative gram-negative bacilli or enterococci, can suppress local host defenses and promote bacterial survival and growth.1 B. fragilis produces a capsular polysaccharide antigen that suppresses complement activation and inhibits leukocyte recruitment and function.9 Anaerobic bacteria produce short-chain fatty acids that can impair the function of neutrophils. Facultative bacteria consume residual oxygen in the microenvironment, permitting the survival and proliferation of obligate anaerobes. Anaerobic bacteria lower the redox potential in the microenvironment, also favoring their growth. Aerobic and anaerobic bacteria can enhance the growth of other species by providing crucial nutrients or the producing enzymes that inactivate antibiotics.
In some respects, bacteria have evolved to take advantage of host defenses. As an example, bacterial adherence to colonocytes and bacterial growth is enhanced by physiologic concentrations of norepinephrine, which is secreted as part of the counter-regulatory response to stress, as well as being administered as an exogenous drug to promote arteriolar constriction and increase myocardial contractility.10
Peritonitis
Peritonitis can be classified as primary, secondary, or tertiary. Most critically ill patients with intraabdominal infection have secondary or tertiary peritonitis. The bacteriology characteristic of these classes of peritonitis is shown in Table 105-1.
TABLE 105-1 Microbiology of Intraabdominal Infection
| Primary (Monomicrobial) | Secondary (Polymicrobial) | Tertiary (Polymicrobial) |
|---|---|---|
| Escherichia coli | Bacteroides fragilis group | Acinetobacter spp. |
| Enterococcus spp. | Clostridium spp. | Enterobacter spp. |
| Klebsiella spp. | E. coli | Enterococcus spp. |
| Streptococcus pneumoniae | Klebsiella spp. | Pseudomonas spp. |
| Other anaerobes | Staphylococcus spp. | |
| Staphylococcus epidermidis | ||
| Streptococcus spp. | ||
| Candida spp. |
Device-associated peritonitis is a variant of primary peritonitis that also almost always is monomicrobial. The great majority of cases occur with chronic ambulatory peritoneal dialysis (CAPD) catheters, which become infected as often as once per year of dialysis.11 The most common pathogens are Staphylococcus aureus and species of Pseudomonas and Candida. Catheter removal is usually necessary to eradicate these infections, especially when caused by P. aeruginosa or Candida spp. Although rare, recurrent CAPD-related peritonitis due to methicillin-resistant S. aureus (MRSA) has been associated with the emergence of vancomycin-resistant strains after treatment with multiple courses of vancomycin.12
Secondary peritonitis follows perforation of a hollow gastrointestinal viscus. The vast majority of cases are community acquired. Appendicitis is the most common cause, and the polymicrobial bacterial flora typically are highly susceptible to antibiotics. Thorough microbiologic analysis of a carefully collected specimen of purulent peritoneal fluid from a patient with secondary peritonitis yields an average of five organisms. B. fragilis the most commonly isolated obligate anaerobe, and E. coli is the most commonly isolated facultative organism. Less common isolates include Enterococcus spp., Candida spp., Clostridium spp., and P. aeruginosa. These uncommon isolates do not need to be covered by the antibiotic regimen if the patient was previously healthy and does not have comorbid conditions that increase the risk for an adverse outcome. Early operative source control, combined with a short course of broad-spectrum antibiotics, are curative in more than 85% of all cases and more than 90% of appendicitis cases.13 Most cases of community-acquired peritonitis do not result in severe illness, and these cases seldom require care in an intensive care unit (ICU).
Tertiary peritonitis describes recurrent or persistent intraabdominal infection after failure of more than one source control procedure to control the infection.14–16 The flora usually include one or more strains of staphylococci (often methicillin-resistant S. epidermidis or MRSA) and Enterococcus spp., Candida spp., or Pseudomonas spp.17–19 It is debated whether tertiary peritonitis represents invasive infection or permissive colonization of the peritoneal cavity in the face of devastated host defenses. The notion that host defenses are compromised is supported by the observation that fluid collections are often poorly localized and serosanguineous rather than purulent. Cases of tertiary peritonitis are fortunately uncommon, but class I data regarding management are lacking.
Adjuvants
Adjuvant substances act to decrease the bacterial inoculum necessary for infection. Adjuvants can increase virulence or interfere with host defenses and invariably are present to some degree in every patient with gastrointestinal perforation. Common adjuvants include ascites, blood, fibrin, bile, urine, chyle, pancreatic juice, and platelets.20 The most important adjuvant is blood. Hemoglobin, fibrin, and platelets all impair host defenses, and iron, which is essential for bacterial growth, also depresses phagocyte function. Fibrin promotes bacterial trapping and abscess formation and can sequester bacteria from neutrophils. Bile salts impair host defenses and are toxic to neutrophils.21 Pancreatic enzymes can be activated by bacterial infection, producing necrotic tissue that is an excellent culture medium.
 At-Risk Patient
At-Risk Patient
Fortunately, most patients with intraabdominal infection are not so sick as to require care in an ICU. In a population-based study of hospital discharges for peritonitis, severe sepsis developed in only 11% of cases (Table 105-2) but increased the mortality risk by 19-fold.2 Similarly, only about 15% of patients enrolled in clinical trials of antimicrobial therapy for secondary peritonitis have an APACHE II score above 15 points.22
TABLE 105-2 Risk Factors for Severe Sepsis in Patients with Intraabdominal Infections
| Parameter | Relative Risk | 95% Confidence Intervals |
|---|---|---|
| Age (Years) | ||
| <20 | 1.0 | |
| 20-39 | 1.4 | 0.8-2.5 |
| 40-59 | 3.2 | 1.8-5.6 |
| 60-79 | 4.6 | 2.6-8.0 |
| >79 | 6.5 | 4.7-11.8 |
| Site | ||
| Appendix | 1.0 | |
| Gallbladder | 2.7 | 1.9-3.8 |
| Colon | 3.9 | 2.6-5.8 |
| Stomach/duodenum | 6.9 | 4.6-10.3 |
| Small bowel | 9.0 | 6.1-13.4 |
| Extent | ||
| Localized | 1.0 | |
| Abscess | 1.2 | 0.8-1.8 |
| Diffuse | 1.5 | 1.1-1.9 |
| Comorbidities | ||
| Congestive heart failure | 1.2 | 1.0-1.6 |
| Stroke | 1.8 | 1.2-2.7 |
| Liver dysfunction | 2.0 | 1.4-2.8 |
| Renal dysfunction | 2.0 | 1.4-2.9 |
Data from Anaya and Nathens.2
Some patients with community-acquired secondary peritonitis have critical illness as a result of delayed presentation, immunosuppression, or extremes of age. However, most patients with critical illness have hospital-acquired peritonitis (Table 105-3). The leading causes of hospital-acquired peritonitis are gastrointestinal anastomotic dehiscence and splanchnic ischemia due to various causes including hypovolemia, distributive shock, atheroembolism, and thromboembolism. Hospital-acquired peritonitis is usually polymicrobial, and commonly cultured organisms include Enterococcus spp., Candida spp., Pseudomonas aeruginosa, and other antibiotic-resistant organisms such as MRSA.14,18,19
TABLE 105-3 Clinical Factors Predicting High-Risk Intraabdominal Infection
Data from Pieracci et al.13 and Solomkin et al.18
The frequency of intraabdominal infection encountered in a particular ICU is variable. Surgical ICUs that care for patients with multiple trauma or following emergency surgery are likely to have more cases of intraabdominal infection than medical ICUs. Surgical patients that have required an operation for source control or have a postoperative secondary nosocomial infection account for 25% to 40% of patients with severe sepsis. However, units with a low prevalence must be equally vigilant in their surveillance and assessment, because a missed intraabdominal infection is almost always fatal.23
When patients with intraabdominal infection are critically ill, mortality exceeds 25%. The risk of failure increases with increasing severity of illness, inadequate empirical antibiotic therapy, delayed surgical therapy, and failure of source control.14,18 Most clinical failures are not associated with multidrug-resistant pathogens, although some data suggest that resistant pathogens cause clinical failure in cases of postoperative peritonitis.24,25
 Spectrum of Disease Causing Critical Illness
Spectrum of Disease Causing Critical Illness
Abscess Of Solid Organs
Treatment of liver abscesses should be individualized. A source of origin should be sought and treated. Antibiotics are mandatory, and a prolonged course for more than 14 days may be necessary. If feasible, based upon the size and location of the abscess, percutaneous drainage always should be attempted.26,27 Operative drainage may be required for abscesses that cannot be drained percutaneously. Overall mortality rate is approximately 25% but is higher for patients with multiple abscesses that are too small to drain.28
Splenic abscesses are uncommon and are the result of hematogenous or local contamination. Hematologic sources include endocarditis, urinary tract infections, pneumonia, osteomyelitis, otitis, mastoiditis, and pelvic infections. Splenic abscesses have been reported with other systemic infections including typhoid, paratyphoid, malaria, and candidiasis. Direct extension from adjacent infections of the pancreas, retroperitoneum, subdiaphragmatic spaces, and diverticulitis can involve the spleen. Systemic disorders such as hemoglobinopathies or sickle cell disease, can cause splenic infarction. Devitalized splenic tissue resulting from trauma, infarction, or embolization can become infected and evolve into splenic abscesses.29
Acute Acalculous Cholecystitis
In contrast to cholecystitis due to gallstones, the etiology of acute acalculous cholecystitis is gallbladder ischemia; infection of the organ occurs secondarily.30 Although acute acalculous cholecystitis can complicate many illnesses, splanchnic hypoperfusion is the common feature. Risk factors in medical patients include congestive heart failure, diabetes mellitus, abdominal vasculitis, and malignancy (including after bone marrow transplantation). Acalculous cholecystitis is more common in surgical patients and can occur following burns, trauma, cardiopulmonary bypass, biliary instrumentation, and emergency aortic surgery.30
The diagnosis of acute acalculous cholecystitis can be challenging, and a high index of suspicion is required. Prompt diagnosis and therapy are necessary, as the disease can be fulminant. Necrosis of the gallbladder occurs in 50% of patients, and perforation of the gallbladder occurs in 20%. Fever and hyperbilirubinemia are common associated findings.29 Serum transaminase and alkaline phosphatase levels also may be elevated. When signs and symptoms can be localized to the right upper quadrant, the differential diagnosis includes gastroduodenal perforation, acute pancreatitis, right colonic ischemia, and acute hepatitis.
Bedside ultrasonography is favored for the diagnosis of acute acalculous cholecystitis; the most accurate diagnostic features are gallbladder wall thickness greater than 3.5 mm and presence of pericholecystic fluid. Computed tomography (CT) is equally accurate and can be utilized when there are no localizing findings and the patient is a candidate for intrahospital transport. Hepatobiliary scintigraphy is not useful to identify or exclude acute acalculous cholecystitis, owing to a high incidence of false-positive findings that result in part from a lack of dietary stimulus for gallbladder contraction. Coadministration of morphine sulfate, which increases biliary hydrostatic pressure, can promote filling of the gallbladder and increase diagnostic accuracy of hepatobiliary scintigraphy.31
Ischemic Colitis And Enteritis
Intestinal ischemia is a dangerous and relatively common complication of critical illness that can progress within hours to gangrene, perforation, and generalized peritonitis.32 The splanchnic circulation is especially vulnerable to low cardiac output, particularly when the cardiac index is less than 2 L/min/m2. Most cases are caused by nonocclusive ischemia; the origin is often multifactorial, including hypovolemia, shock, and administration of vasopressors. A number of other causes have been identified. Acquired protein C/protein S deficiency induces a hypercoagulable state that has been associated with mesenteric arterial and venous thrombosis. Chronic atrial fibrillation or dilated cardiomyopathy can lead to mesenteric arterial thromboembolism. Arteriography can cause cholesterol embolization from dislodgement of an atherosclerotic plaque. Intestinal obstruction also must be considered, and this diagnosis may not be obvious with partial or proximal obstructions.
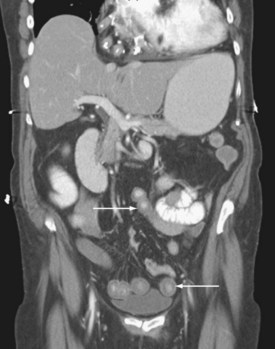
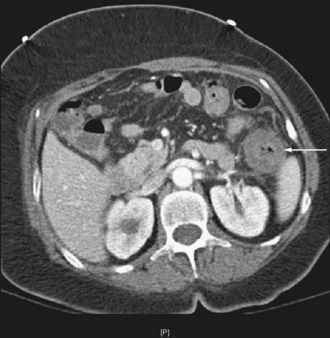
The pattern of injury with intestinal ischemia is variable depending on the mechanism, the presence of heart disease, and the status of the collateral circulation via the celiac and inferior mesenteric arteries. Large thrombi usually occlude the superior mesenteric artery where it narrows just distal to origin of the middle colic artery. The first 30 to 45 cm of small bowel and the left colon may be spared. Smaller emboli are more likely to infarct the small bowel and possibly the ascending colon (Figure 105-1); the distribution can be patchy. Nonocclusive ischemia classically occurs in watershed areas of the mesenteric circulation where collateral vessels bridge the two arterial distributions. A typical site is the splenic flexure of the colon at the watershed junction of the superior and the inferior mesenteric arteries. Although any segment of intestine can be affected by nonocclusive ischemia, the cecum (the point farthest from inferior mesenteric artery collaterals) and the left colon are most likely to be affected (Figure 105-2). The left colon is particularly vulnerable after abdominal aortic operations, especially when the inferior mesenteric artery has been ligated during the procedure.
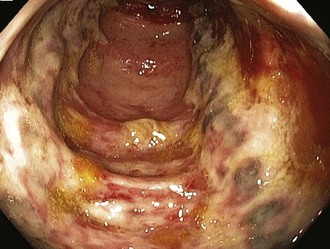
Given the propensity for left colonic involvement, lower endoscopy at the bedside is usually the first diagnostic modality to be employed, but a number of pitfalls should be recognized. Flexible sigmoidoscopy is simple and safe but may miss ischemia at or proximal to the splenic flexure; accordingly, colonoscopy is preferred (Figure 105-3). Endoscopy visualizes only the mucosa and can underestimate the extent of disease. CT and CT angiography are increasingly useful diagnostically and have largely supplanted use of formal arteriography (Figure 105-4).
< div class='tao-gold-member'>

Full access? Get Clinical Tree