Fig. 20.1
Stimulating 14-G Coudé needle and multipolar electrode
This needle has been used in five patients undergoing peripheral neuromodulation implantation and its easy steerability and stimulation at the introducer tip allows passage of the electrode at the exact location and depth required to achieve optimal coverage of the painful area.
The terminology is still under debate as a number of different terms have been used to describe this technique. We use the term “subcutaneous targeted stimulation” as it uses the principle that the stimulation is targeted at a specific epicentre of pain and it is not designed to use the electrical field itself to cover the painful area [48]. The feeling of stimulation is much larger than the electrical field itself. Other terms used are peripheral nerve field stimulation and subcutaneous electrical neurostimulation [49].
The Use of Non-invasive External Neuromodulation
The application of low frequency non-invasive stimulation using an external nerve mapping probe is termed external neuromodulation and it allows mapping of potential peripheral targets for neuromodulation without needle insertion. The probe (Neuro-Trace, HDC Corporation, Milpitas, California, USA, Pajunk GmbH, Geisingen, Germany) was introduced to map nerve location for regional anaesthesia, but its adoption in using it to treat neuropathic pain states has led to its use as a method of stimulating nerves or the epicentre of pain in a non-invasive manner [16, 50]. This stimulation is at a frequency of 2 Hz and the ball-shaped probe is placed over the skin using known surface landmarks of nerve anatomy or at the point of greatest pain for non-nerve pain distributions. Figure 20.2 shows the probe being used over the inguinal region.
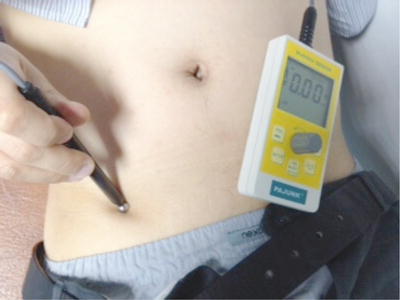

Fig. 20.2
Application of external neuromodulation over ilioinguinal nerve for groin pain
The amplitude of stimulation is increased so that paraesthesia is perceived. The procedure can be performed on an outpatient basis and it is important to emphasise that this technique should not be confused with TENS. The electrical field from the EN is deeper and more focused as it has a higher current density [16].
Its use can be both in screening for whether the patient’s pain is suitable for other peripheral neuromodulation techniques or if analgesia is prolonged then this can be used as a single modality alone and the patient can be provided with their machine and be taught to self-administer [51].
With an increasing variety of techniques available, our practice is to use the various techniques in a logical manner from the least invasive at the beginning of treatment to invasive implanted therapies for the most refractory pain conditions [52].
Stage I: The application of External Neuromodulation allows mapping of the nerves or target that may be amenable for stimulation. If analgesia is prolonged then this can be the sole therapy and patients can self-administer.
Stage II: Direct stimulation of the peripheral nerve or at the epicentre of pain using an insulated needle for approximately 5 min. Again, this provides further information on nerve mapping and what the best targets for stimulation would be for future treatment. Unlike external neuromodulation, this is usually not the sole therapy alone as repeat stimulation cannot be self-administered.
Stage III: Trial of stimulation. Once an appropriate area is mapped out with the above techniques, a trial of stimulation using either a monopolar stimulating catheter or standard spinal cord stimulator multipolar electrodes can be commenced. The monopolar lead (Stimulong, Pajunk) is inexpensive, but multipolar electrodes are useful if the area of stimulation is large or monopolar stimulation is inconclusive [53].
Stage IV: If pain relief from the trial is greater than 50 % and no adverse effects reported then we proceed to permanent insertion of multipolar leads to an internal implanted pulse generator.
A function of EX Stimulation in diagnosis and treatment of abdominal visceral pain has not been fully explored as yet; however, its success in pain states, such as refractory angina and neuropathic pain, may suggest a role in the future.
Lumbar Sympathetic Chain Stimulation: Another Target for Renal Pain
LPHS presents with flank pain and micro and macroscopic haematuria. Its aetiology is unknown and poorly understood, and diagnosis is one of exclusion. Renal function is normal and the aim is symptom control as there is no progression to chronic renal failure.
These patients have severe loin pain which is usually refractory to conventional treatments. One potential target of stimulation is the lumbar sympathetic chain as it was believed that many of the nociceptive afferents were autonomic. Four cases of loin pain haematuria syndrome have been successfully treated with stimulation of the lumbar sympathetic chain [24].
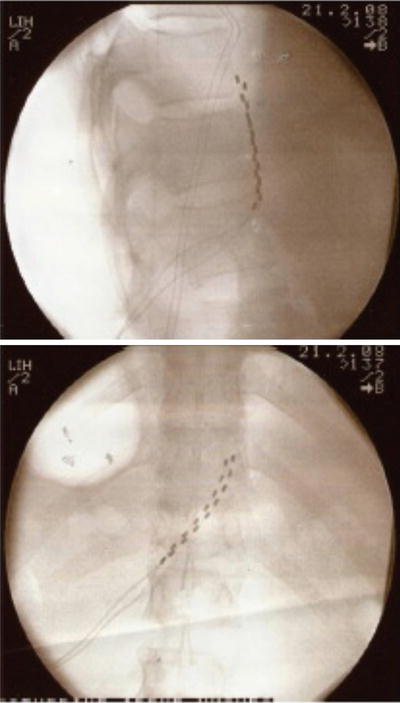
As with the case of splanchnic stimulation, the initial approach involved using low-frequency stimulation of the lumbar sympathetic chain at L3 with a stimulating needle at 2 Hz for 5 min. This allowed the authors to determine if sympathetic chain stimulation was effective in relieving the pain. All patients developed pleasant stimulation covering all of their pain and after 5 min of stimulation two of the patients had 24 h of complete pain relief. After this, a trial of stimulation using monopolar electrodes was performed by inserting them at the level of the anterior border of the L3 vertebral body. In three patients the trial achieved full pain relief. The monopolar electrodes were chosen for their simplicity of insertion and economy. Full implantation in two patients with multipolar electrodes (Pisces quad, Medtronic Inc., Minnesota, USA) provided effective long-term pain relief. Insertion of these electrodes was performed by using a 14-G long Tuohy needle as an introducer inserted to the anterior border of the L3 and L4 vertebral body. The leads were connected to an implanted pulse generator in the anterior abdominal wall. Figure 20.3 shows the position of the leads. A role of this interesting and attractive new approach has to be evaluated further.
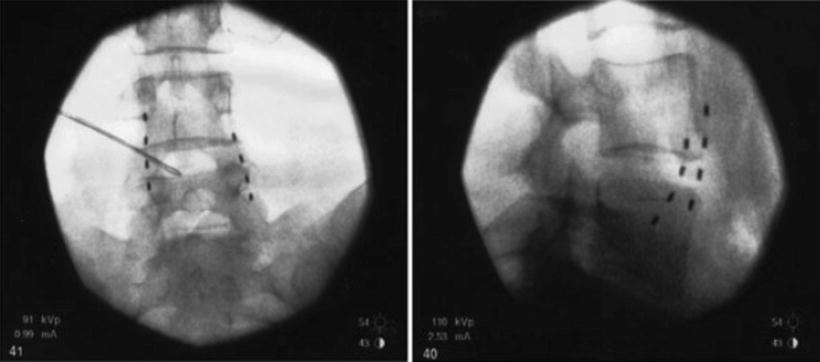

Fig. 20.3
Lead position for lumbar sympathetic chain stimulation
Following permanent implantation in these two patients, they continued with complete pain relief and stimulation was required only two or three times daily. The third patient had 7 months from a trial period of stimulation and full implantation was thus delayed and the fourth patient is awaiting implantation.
Stimulation of Splanchnic Nerves
The first case report of peripheral stimulation of the splanchnic nerves is a 36-year-old patient who had chronic pancreatitis and severe right upper quadrant abdominal pain [54]. This pain was exacerbated by food and she had undergone cholecystectomy, endoscopic incision of the sphincter of Oddi, and a Roux-en-Y procedure. She continued to have persistent pain and a celiac plexus block with alcohol only provided 3 months of pain relief. She required large doses of fentanyl daily and a Whipple’s procedure was proposed as a last resort but she refused.
As external Neuromodulation would not be possible, it was decided that the first approach would to perform stimulation of the celiac plexus. This was performed by inserting two 150 mm stimulating needles at L1 to the celiac plexus and stimulation at 2 Hz for 5 min at amplitude that produced a pleasant sensation in the abdomen. Her pain scores dropped from 9 out of 10 on a VAS scale to 0 out of 10 for the next 48 h.
This encouraging result was followed by insertion of temporary monopolar electrodes as a trial of stimulation. Two monopolar electrode catheters were inserted under fluoroscopic control to the celiac plexus at L1. Stimulation was again at 2 Hz and excellent pain relief (VAS 0/10) was achieved during the trial. Only 10 min of stimulation was performed every 12 h.
Permanent implantation was followed and this was associated with some technical issues. As implantation required the use of a large diameter introducer to accommodate the multipolar electrodes avoidance of vascular puncture is critical. Two 14-G 150 mm Tuohy needles were inserted under fluoroscopy to lie adjacent to the edge of the L1 anterior border. To prevent vascular puncture, the needles were not placed beyond this point. As it was not possible to place the leads at the celiac plexus, a guide wire was inserted and this followed the anterior border of the T11 and T12 vertebral bodies. The Tuohy needle was removed and an introducer railroaded over the guide wires. This allowed placement of two octopolar leads at the anterior border of the T11 and T12 bodies. Stimulation at 2 Hz produced pleasant abdominal stimulation covering the painful areas. The leads were connected to an extension and connected to an implanted pulse generator (EON, St Jude Medical Inc., USA) placed at the anterior abdominal wall.
Stimulation was only required for up to 6 h daily and she was able to reduce her fentanyl from 225 to 12.5 μg/h and stop the use of breakthrough analgesia. Her weight started to increase and she resumed employment.
This case illustrated the use of the potential of peripheral stimulation at the splanchnic nerves for VAP. As this treatment still requires validation with more patients and eventually randomised controlled trials we have to remain cautious but the long-term lead stability remains good and it is a possible alternative to spinal cord stimulation.
Using a Combined Peripheral and Spinal Cord Stimulation Approach
The peripheral or spinal cord approaches do not have to be mutually exclusive and many cases of combined approaches have been described in the literature. In this case, we used this dual approach in a 63-year-old lady with severe abdominal and flank pains secondary to chronic non-alcoholic pancreatitis. She had a poor response to conventional medical management including opioids, celiac and splanchnic nerve blockade. A trial of splanchnic nerve neuromodulation was successful using two monopolar electrodes and she proceeded to full implantation. Two quadripolar electrodes were inserted at the level of T11 and T12 in a similar manner to the case above and in addition, a octopolar electrode was inserted in the epidural space to a level of T10 (Fig. 20.5.). An EON mini was used as the implantable pulse generator. This provided excellent pain relief and she uses the stimulator three to four times a week for approximately 4 h at a time. The leads remained stable at 17-month follow-up (Fig. 20.5).
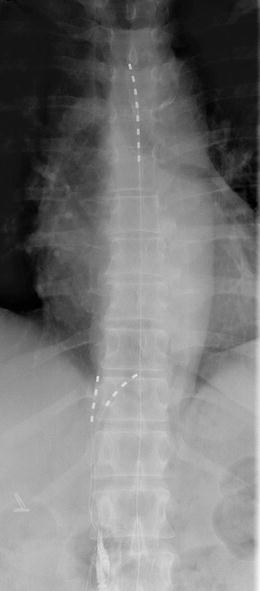

Fig. 20.5
Combined SCS and splanchnic leads in situ
Current Indications for Using Peripheral Neuromodulation and Future Applications
The indications for using peripheral stimulation for abdominal visceral pain are similar to that of other Neuromodulation techniques. Pain relief has been demonstrated in chronic pancreatitis, post-surgical abdominal pain and loin pain haematuria syndrome. For pains with a specific nerve distribution or when no dermatological pattern is present the peripheral approach may be successful when spinal cord stimulation is not. However, the techniques can be combined and addition of peripheral electrodes to existing spinal cord stimulator systems can achieve pain relief when use of a single modality is unsuccessful.
Future work in this field must include both innovative approaches to stimulation itself and to produce high-quality evidence base for the various approaches as much of the literature is based on case reports and series at present. It is of note that most peripheral technologies are derived from spinal cord stimulation and regional anaesthesia. Peripheral specific electrode and battery design will improve stimulation coverage and efficacy, battery issues and morbidity.
Conclusion
Peripheral nerve stimulation is amongst the fastest growing aspects of Neuromodulation. Its promise in treating areas of pain that is not possible with spinal cord stimulation and the exciting possibility of treating pain of nociceptive origin justifies its attractions to an increasing number of clinical investigators. The previous restriction of requiring surgical access for placement of implanted electrodes has now been reduced with the development of percutaneous techniques alongside improved imaging technologies.
Despite the advances in peripheral nerve stimulation for painful chronic conditions, its mechanism of action has not been as well studied as for spinal cord stimulation and due to the heterogeneity in techniques and methods of implantation there remains a lack of high-quality evidence in its use. The challenges for the future of peripheral nerve stimulation are not only in simplifying and improving the technical aspects of implantation but also to show that the method itself has a high-quality evidence base to justify it becoming a standard and mainstream treatment for chronic pain.

Full access? Get Clinical Tree