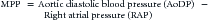Chapter 34 Performance of Cardiopulmonary Resuscitation in Infants and Children
Pediatric cardiac arrest is not a rare event. Approximately 16,000 American children (8-20/100,000 children/year) experience cardiopulmonary arrest each year.1–5 Approximately half of these cardiac arrests occur in-hospital, and about half outside the hospital.5,6 In times past, survival outcomes were not good and many children had severe neurological injury after their arrest event. With advances in resuscitation science and implementation techniques, survival from pediatric cardiac arrest has improved substantially over the past 25 years.7 This chapter focuses on pediatric cardiac arrest, cardiopulmonary resuscitation (CPR), and other therapeutic interventions that have been specifically designed to improve outcomes from pediatric cardiac arrest.
Four Phases of Cardiac Arrest
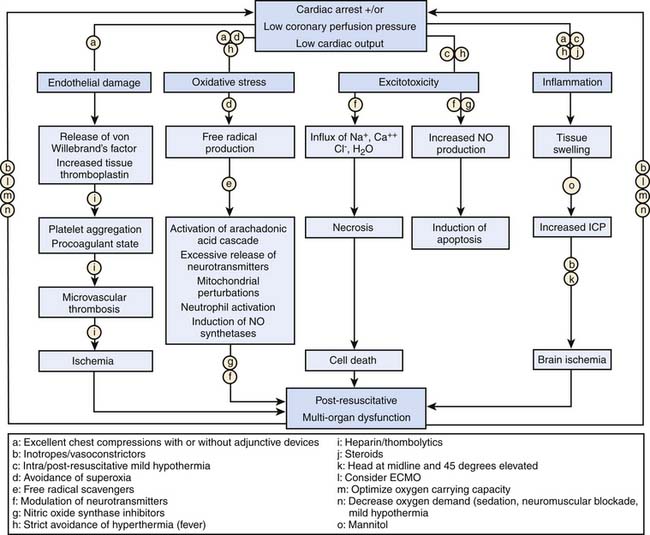
The four distinct phases of cardiac arrest and CPR interventions are (1) prearrest, (2) no flow (untreated cardiac arrest), (3) low flow (CPR), and (4) postresuscitation. Interventions to improve the outcome of pediatric cardiac arrest should optimize therapies targeted to the time and phase of CPR, as suggested in Table 34-1.
Table 34–1 Phases of Cardiac Arrest and Targeted Interventions
| Phase | Interventions |
|---|---|
| Prearrest phase: Protect | |
| Arrest (no-flow): Preserve | |
| Low-flow (CPR): Resuscitate | |
| Long-term |
Prearrest
The prearrest phase refers to relevant preexisting conditions of the child (e.g., neurologic, cardiac, respiratory, or metabolic problems) and precipitating events (e.g., respiratory failure or shock). It is known that pediatric patients who suffer an in-hospital cardiac arrest often have changes in their physiological status in the hours leading up to their arrest event.8,9 Therefore, interventions during the prearrest phase focus on preventing the cardiac arrest, with special attention to early recognition and treatment of respiratory failure and shock. Rapid-response teams or medical emergency teams (METs) are in-hospital emergency teams designed specifically for this purpose. These teams respond to patients on general inpatient units who are at high risk of clinical decompensation and transfer these children to more acute care areas, with the goal to prevent progression to full cardiac arrest. Implementation of pediatric METs has been moderately successful; decreased cardiac arrest frequency and mortality have been demonstrated.10–12 While METs cannot identify all children at risk for cardiac arrest, it seems reasonable to assume that transferring critically ill children to an intensive care unit (ICU) early in their disease process for better monitoring and more aggressive interventions can improve resuscitative care and clinical outcome.
No Flow/Low Flow
In order to improve outcomes from pediatric cardiac arrest, it is imperative to shorten the no-flow phase of untreated cardiac arrest. To that end, it is important to monitor high-risk patients to allow early recognition of the cardiac arrest and prompt initiation of basic and advanced life support. Effective CPR optimizes coronary perfusion pressure and cardiac output to critical organs to support vital organ viability during the low-flow phase. Important tenets of basic life support are push hard, push fast, allow full chest recoil between compressions, and minimize interruptions of chest compression. Achieving optimal coronary perfusion pressure, exhaled carbon dioxide concentration, and cardiac output during the low-flow phase of CPR is consistently associated with an improved chance for return of spontaneous circulation (ROSC) and improved short and long term outcome in both animal and human studies.13–20 For ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT), rapid detection and prompt defibrillation are vital for successful resuscitation. For cardiac arrests resulting from asphyxia and/or ischemia, provision of adequate myocardial perfusion and myocardial oxygen delivery are most important.
Epidemiology of Pediatric Cardiac Arrest
Cardiovascular disease remains the most common cause of disease-related death in the United States, resulting in approximately 1 million deaths per year.21 It is estimated that more than 400,000 Americans will have a cardiac arrest each year, nearly 90% in prehospital settings. While data regarding the incidence of childhood cardiopulmonary arrest are less robust, the best data suggest that about 16,000 American children suffer a cardiac arrest each year (annual incidence: 8 to 20 per 100,000 children per year).1–522 For in-hospital arrests specifically, it is estimated that approximately 2% to 6% of all children admitted to pediatric intensive care units,1,2,23 and 4% to 6% of children admitted to cardiac units will suffer a cardiac arrest.24,25 In short, pediatric cardiac arrest is an important public health problem.
Outcomes from pediatric cardiac arrest have improved significantly over the past 20 years (Table 34-2). Nearly two thirds of children who have an in-hospital cardiac arrest are successfully resuscitated initially (i.e., attain sustained ROSC). Moreover, more than 25% of them will survive to hospital discharge, and many (nearly 75%) will have good neurologic function.1–4,7,25–37 Factors that influence outcome from pediatric cardiac arrest include (1) the preexisting condition of the child, (2) the initial electrocardiographic (ECG) rhythm detected, (3) the duration of no-flow time (the time during an arrest without spontaneous circulation or provision of CPR), and (4) the quality of the life-supporting therapies provided during the resuscitation. With this knowledge, it is no surprise then that out-of-hospital pediatric arrests have worse outcomes compared to in-hospital arrests.3,22,23,30,35,38–44 As many of these out-of-hospital events are not witnessed and bystander CPR is not common (less than 30% of children receive bystander CPR),3 the duration of no-flow time can be prolonged. As a result, less than 10% of these children survive their initial event, and in those that do survive, neurological injury is common. These findings are especially troublesome given that bystander CPR more than doubles patient survival rates.45
Table 34–2 Cardiac Arrest Outcomes for Both In-Hospital and Out-of-Hospital Pediatric Cardiac Arrest
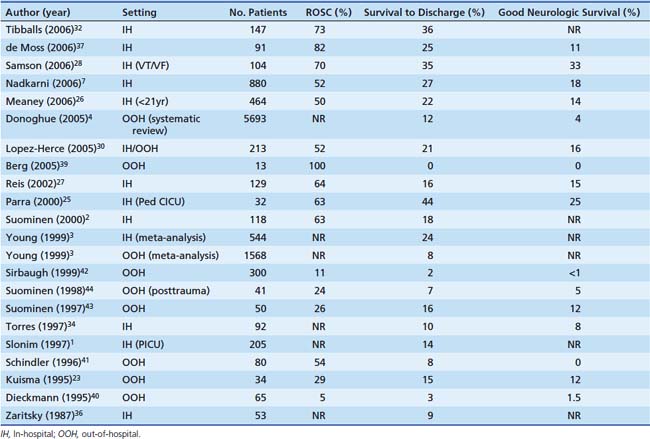
Compared to adults, superior survival rates are documented after pediatric cardiac arrest, specifically after in-hospital events; 27% of children survive to hospital discharge compared with only 17% of adults.7 These findings may be in part due to differences in the initial ECG rhythm detected. While pediatric arrests are less commonly caused by arrythmias, such as ventricular tachycardia or ventricular fibrillation—10% of pediatric arrests versus 25% of adult arrests—the superior pediatric survival rate reflects a substantially higher survival rate among children with asystole or pulseless electrical activity compared with adults (24% vs. 11%). Moreover, the higher survival rate seen in children is mostly attributable to a much better survival rate among infants and preschool age children compared with older children.26 Although this is speculative, the higher survival rates in children may be due to improved coronary and cerebral blood flow during CPR because of increased chest compliance in these younger arrest victims.46,47
Interventions During the Low-Flow Phase: Cardiopulmonary Resuscitation
Airway and Breathing
During the low-flow state of CPR, cardiac output and pulmonary blood flow are approximately 25% of that during normal sinus rhythm; therefore, much less ventilation is necessary for adequate gas exchange from the blood traversing the pulmonary circulation. Moreover, animal and adult data indicate that a rapid rate of assisted ventilation (“overventilation” from exuberant rescue breathing) during CPR is common and can substantially compromise venous return and cardiac output by increasing intrathoracic pressure.48–50 Moreover, these detrimental hemodynamic effects are compounded when one considers the effect of interruptions in CPR to provide airway management and rescue breathing.51–55 While overventilation is problematic, in light of the fact that most pediatric arrests are asphyxial in nature, provision of adequate ventilation is still important. The difference between arrythmogenic and asphyxial arrests lies in the physiology. In animal models of sudden VF cardiac arrest, acceptable PaO2 and PaCO2 persist for 4 to 8 minutes during chest compressions without rescue breathing.56 This is in part because aortic oxygen and carbon dioxide concentrations at the onset of the arrest do not vary much from the prearrest state. As a result, the lungs act as a reservoir of oxygen during CPR, and adequate oxygenation and ventilation can continue without rescue breathing. However, during asphyxial arrest, blood continues to flow to tissues in the prearrest state, resulting in significant arterial and venous oxygen desaturation, elevated lactate levels, and depletion of the pulmonary oxygen reserve. Therefore, at the onset of resuscitation, there is substantial arterial hypoxemia and acidemia. In this circumstance, rescue breathing with controlled ventilation can be lifesaving. In contrast, the adverse hemodynamic effects from overventilation during CPR combined with the interruptions in chest compressions to open the airway and deliver rescue breathing are a lethal combination in certain circumstances such as VT/VF arrests. In short, the resuscitation technique should be titrated to the physiology of the patient to optimize patient outcome.
Circulation
Optimizing Blood Flow During Low-Flow Cardiopulmonary Resuscitation: Push Hard, Push Fast
When the heart arrests and no blood flows to the aorta, coronary blood flow ceases immediately.57 At that point, provision of high-quality CPR (push hard, push fast) is necessary to reestablish flow. The goal during CPR is to maximize the myocardial perfusion pressure (MPP). Related by the following equation:
myocardial blood flow improves as the gradient between AoDP and RAP increases. During downward compression phase, aortic pressure rises at the same time as right atrial pressure with little change in the MPP. However, during the decompression phase of chest compressions, the right atrial pressure falls faster and lower than the aortic pressure, which generates a pressure gradient perfusing the heart with oxygenated blood during this artificial period of “diastole.” Several animal and human studies have demonstrated in both VT/VF and asphyxial models the importance of establishing MPP as a predictor for short term survival outcome (ROSC).19,58–61
Based on the equation above, MPP can be improved by strategies that increase the pressure gradient between the aorta and the right atrium. As an example, the inspiratory impedance threshold device (ITD) is a small, disposable valve that can be connected directly to the tracheal tube or face mask to augment negative intrathoracic pressure during the inspiratory phase of spontaneous breathing and the decompression phase of CPR by impeding airflow into the lungs. Application in animal and adult human trials of CPR has established the ability of the ITD to improve vital organ perfusion pressures and myocardial blood flow51,62–65; however, in the only randomized trial during adult CPR, mortality benefit was limited to the subgroup of patients with pulseless electrical activity.66 Additional evidence that augmentation of negative intrathoracic pressure can improve perfusion pressures during CPR comes from the active compression-decompression device (ACD). The ACD is a handheld device that is fixed to the anterior chest of the victim by means of suction—think household plunger—that can be used to apply active decompression forces during the release phase, thereby creating a vacuum within the thorax. By actively pulling during the decompression phase, blood is drawn back into the heart by the negative pressure.67 Animal and adult studies have demonstrated that the combination of ACD with ITD acts in concert to further improve perfusion pressures during CPR compared to ACD alone.63 In the end, while novel interventions such as the ITD and ACD are promising to improve blood flow during CPR, the basic tenants of “push hard, push fast, minimize interruptions, and don’t overventilate” are still the dominate factors to improve blood flow during CPR and chance of survival.
Chest Compression Depth
The pediatric chest compression depth recommendation of at least one-third anterior-posterior chest depth (approximately 4 cm in infants and 5 cm in children) is based largely upon expert clinical consensus, using data extrapolated from animal, adult, and limited pediatric data. Recently, Maher et al. published data from a case series of infants postcardiac surgery associating arterial blood pressure with qualitative chest compression depths. In this small study of 6 infants, chest compressions targeted to one-half anterior-posterior chest depth imparted improved systolic blood pressures compared to those at one-third anterior-posterior chest depth.68 While a small series with qualitatively estimated chest compression depths, this is the first study to collect actual data from children supporting the existing chest compression depth guidelines. On the contrary, two recent studies using computer-automated tomography69,70 suggest that depth recommendations based on a relative (%) anterior-posterior chest compression depth are deeper than those recommended for adults, and that a depth of one-half anterior-posterior chest depth is unattainable in most children. Future studies that collect data from actual children and that associate quantitatively measured chest compression depths with short- and long-term clinical outcomes (arterial blood pressure, end-tidal carbon dioxide, return of spontaneous circulation, survival) are needed.
Compression/Ventilation Ratios
The amount of ventilation provided during CPR should match, but not exceed, perfusion and should be titrated to the amount of circulation during the specific phase of resuscitation as well as the metabolic demand of the tissues. Therefore during the low-flow state of CPR when the amount of cardiac output is roughly 25% of normal, less ventilation is needed.71 However, the best ratio of compressions to ventilations in pediatric patients is largely unknown and depends on many factors including the compression rate, the tidal volume, the blood flow generated by compressions, and the time that compressions are interrupted to perform ventilations. Recent evidence demonstrated that a compression/ventilation ratio of 15:2 delivers the same minute ventilation and increases the number of delivered chest compressions by 48% compared to CPR at a compression/ventilation ratio of 5:1 in a simulated pediatric arrest model.72,73 This is important because when chest compressions cease, the aortic pressure rapidly decreases and coronary perfusion pressure falls rapidly.57 Increasing the ratio of compressions to ventilations minimizes these interruptions, thus increasing coronary blood flow. These findings are in part the reason the American Heart Association (AHA) now recommends a pediatric compression/ventilation ratio of 15:2.
Duty Cycle
In a model of human adult cardiac arrest, cardiac output and coronary blood flow are optimized when chest compressions last for 30% of the total cycle time (approximately 1:2 ratio of time in compression to time in relaxation).74 As the duration of CPR increases, the optimal duty cycle may increase to 50%. In a juvenile swine model, a relaxation period of 250 to 300 milliseconds (duty cycle of 40% to 50% at a compression rate of 120/min) correlates with improved cerebral perfusion pressures compared with shorter duty cycles of 30%.75
Circumferential Versus Focal Sternal Compressions
In adult and animal models of cardiac arrest, circumferential (vest) CPR has been demonstrated to improve CPR hemodynamics dramatically.76 In smaller infants, it is often possible to encircle the chest with both hands and depress the sternum with the thumbs, while compressing the thorax circumferentially (thoracic squeeze). In an infant animal model of CPR, this “two-thumb” method of compression with thoracic squeeze resulted in higher systolic and diastolic blood pressures and a higher pulse pressure than traditional two-finger compression of the sternum.77
Open-Chest Cardiopulmonary Resuscitation
Excellent standard closed-chest CPR generates cerebral blood flow that is approximately 50% of normal. By contrast, open-chest CPR can generate cerebral blood flow that approaches normal. Whereas open-chest massage improves coronary perfusion pressure and increases the chance of successful defibrillation in animals and humans,78–80 performing a thoracotomy to allow open-chest CPR is impractical in many situations. A retrospective review of 27 cases of CPR following pediatric blunt trauma (15 with open-chest CPR and 12 with closed-chest CPR) demonstrated that open-chest CPR increased hospital cost without altering rates of ROSC or survival to discharge. However, survival in both groups was 0%, indicating that the population may have been too severely injured or too late in the process to benefit from this aggressive therapy.81 Earlier institution of open-chest CPR may warrant reconsideration in selected special resuscitation circumstances.
Medications Used to Treat Cardiac Arrest
Vasopressors
Epinephrine (adrenaline) is an endogenous catecholamine with potent α- and β-adrenergic stimulating properties. The α-adrenergic action (vasoconstriction) increases systemic and pulmonary vascular resistance. The resultant higher aortic diastolic blood pressure improves coronary perfusion pressure and myocardial blood flow even though it reduces global cardiac output during CPR. Adequacy of myocardial blood flow is a critical determinant of ROSC. Epinephrine also increases cerebral blood flow during CPR because peripheral vasoconstriction directs a greater proportion of flow to the cerebral circulation.82–84 However, recent evidence suggests that epinephrine can decrease local cerebral microcirculatory blood flow at a time when global cerebral flow is increased.85 The β-adrenergic effect increases myocardial contractility and heart rate and relaxes smooth muscle in the skeletal muscle vascular bed and bronchi; however, the β-adrenergic effects are not observed in the peripheral vascular beds secondary to the high dose used in cardiac arrest. Epinephrine also increases the vigor and intensity of VF, increasing the likelihood of successful defibrillation.
High-dose epinephrine (0.05 to 0.2 mg/kg) improves myocardial and cerebral blood flow during CPR more than standard-dose epinephrine (0.01 to 0.02 mg/kg) in animal models of cardiac arrest and may increase the incidence of initial ROSC.86,87 Administration of high-dose epinephrine, however, can worsen a patient’s postresuscitation hemodynamic condition. Retrospective studies indicate that use of high-dose epinephrine in adults or children may be associated with a worse neurologic outcome.88,89 A randomized, controlled trial of rescue high-dose epinephrine versus standard-dose epinephrine following failed initial standard-dose epinephrine in pediatric in-hospital cardiac arrest demonstrated a worse 24-hour survival in the high-dose epinephrine group (1/27 vs. 6/23, P < .05).90 Based on these clinical data, high-dose epinephrine cannot be recommended routinely for either initial or rescue therapy.
Vasopressin is a long-acting endogenous hormone that acts at specific receptors to mediate systemic vasoconstriction (V1 receptor) and reabsorption of water in the renal tubule (V2 receptor). The vasoconstriction is most intense in the skeletal muscle and skin vascular beds. Unlike epinephrine, vasopressin is not a pulmonary vasoconstrictor. In experimental models of cardiac arrest, vasopressin increases blood flow to the heart and brain and improves long term survival compared with epinephrine. However, vasopressin can decrease splanchnic blood flow during and following CPR and can increase afterload in the postresuscitation period.91–95 Adult randomized controlled trials suggest that outcomes are similar after use of vasopressin or epinephrine during CPR.96,97 During pediatric arrest, a case series of four children who received vasopressin during six prolonged cardiac arrest events suggested that the use of bolus vasopressin may result in ROSC when standard medications have failed.98 However, a more recent retrospective study of 1293 consecutive pediatric arrests from the National Registry of CPR (NPCRP) found that vasopressin use, while infrequent (administered in only 5% of events), was associated with a lower likelihood of ROSC. Therefore, it is unlikely that vasopressin will replace epinephrine as a first-line agent in pediatric cardiac arrest. However, the available data suggest that its use in conjunction with epinephrine may deserve further investigation.
Calcium
Calcium is used frequently in cases of cardiac arrest, despite the lack of evidence for efficacy when it is administered routinely during resuscitation attempts. In the absence of a documented clinical indication (i.e., hypocalcemia, calcium channel blocker overdose, hypermagnesemia, or hyperkalemia), administration of calcium does not improve outcome from cardiac arrest.37,99–107 To the contrary, three pediatric studies have suggested a potential for harm, as routine calcium administration was associated with decreased survival rates and/or worse neurological outcomes.37,99,100

Full access? Get Clinical Tree