Fig. 5.1
Danger sensing mechanisms at trauma. DAMPs danger associated molecular patterns, PAMPs pathogen associated molecular patterns
Cytokines are polypeptides that are produced from a variety of cells such as monocytes/macrophages and T-helper lymphocytes. Interleukin-1 (IL-1), IL-6, IL-8, IL-10 and tumor necrosis factor (TNF) are cytokines that transmit signals between cells thus enhancing their communication and playing an important role in the development of SIRS and MODS. In particular, TNF activates cells such as NK-cell and macrophages and induces apoptosis [48]. It leads to thromboxane A2, prostaglandin, selectin, platelet activation factor, and intracellular adhesion molecules production. It exerts its effects via remote and local action. Up-to-date effective inhibition of TNF has not been successful although blocking it might work in septic patients [49].
Interleukin 1 (IL-1) is another cytokine involved in signaling during major trauma. Its secretion pathway has not been fully understood so far. It induces T-cell and macrophage application and activates a cascade that leads to transcription of many different pro-inflammatory cytokines [35]. Interleukin 6 (IL-6) is the most extensively studied cytokine that is promptly detectable after major trauma (within hours). Its plasma half-life and the consistent pattern of expression have established it as the most widely studied pro-inflammatory molecule [50]. It regulates growth and differentiation of lymphocytes, and activates NK-cells and neutrophils. At the same time it inhibits the apoptosis of neutrophils having therefore a role both as a pro-inflammatory and anti-inflammatory protein [51, 52]. In animal models it has been shown that blockade of IL-6 increases survival [53]. It has also been proved that a certain cut-off of 200 pg/dl could effectively be used as a diagnostic and predictive means of SIRS and later complications in the clinical setting [50]. IL-8 belongs to chemotactic cytokines which are called chemokines and act as chemoattractants. Depending on its concentration gradient IL-8 can act as an angiogenic factor and a very effective chemmoattractant. It activates the neutrophills as well as lymphocytes, monocytes, endothelial cells, and fibroblasts [54].
The physiologic response to trauma is a multifaceted phenomenon that can be influenced and modified by several different variables. It has been shown to be gender dependent and the role of sex hormones in the course of postinjury immune response is now accepted. In animal models, males and ovariectomized females exhibit a more intense alteration in immune function following hemorrhage after trauma [55]. Females have demonstrated a relative better response to traumatic shock and hemorrhage compared to males [56, 57]. In a retrospective analysis of 43,394 patients Haider et al. [58] demonstrated an increased survival rate among severely injured females (ISS > 16 and systolic blood pressure <90 mmHg) between 13 and 64 years of age who sustained shock after trauma. Asimilar difference has not in mortality was observed in younger and elderly patients, that is, patients not affected by sex hormones a finding that suggests the possible contribution of hormones to sex-based mortality after shock following trauma. Furthermore, a recent large retrospective analysis of 244,371 adult patients with blunt trauma and ISS > 16 revealed that Asian females had a 40 % lower risk of mortality relative to Asian males suggesting that in Asian race there is a sex-based outcome difference [59]. These findings underpin the racial and gender-based differences in outcome after significant trauma.
In recent years there is a growing body of evidence that posttraumatic complications could be influenced by the genetic background (genotype) of each patient and that genotyping might be helpful in detecting complication prone polytrauma patients [33, 60–63]. The application of genomic studies to practice is a promising evolving field that will probably affect our perspectives and management of polytrauma in the near future and will allow a more personalized approach to our patients [63, 64]. Bronkhorst et al. [65] performed genotyping of single-nucleotide polymorphisms in Toll-like receptor and cluster of differentiation 14 (CD14) genes in 219 polytraumatized patients and detected that the presence of a specific type of TLR2 genotype increased the risk of developing gram-positive infection and SIRS. Similarly, specific single nucleotide polymorphisms in the lectin pathway have been found to correlate with increased risk of developing sepsis, SIRS, and septic shock in polytrauma patients [66]. Additionally, three single nucleotide polymorphisms and haplotypes were found to be associated with increased production of high-mobility group box protein 1 in the peripheral blood and the increased risk of MODS and sepsis in trauma patients was suggested [67]. An overview of the most recent studies investigating the association of specific gene polymorphism with the development of complications in polytrauma patients is presented in Table 5.1.
Table 5.1
Recent studies investigating the association of specific gene polymorphism with the development of complications in polytrauma patients
Authors | Year | Gene | Number of patients | Conclusion |
|---|---|---|---|---|
Bronkhorst et al. [66] | 2013 | MBL2, MASP2, and FCN2in the lectin pathway | 219 | SNPs in the lectin pathway predispose polytrauma patients for SIRS, infectious complication, septic shock, and positive culture findings |
Bronkhorst et al. [65] | 2013 | TLR/CD14 | 219 | SNPs in TRL2 T-16934A and TRL9 T-1486C increased, decreased the risk of developing posttraumatic infectious complications, respectively. Early genotyping might be useful to detect the patients prone to develop complications |
Zeng et al. [68] | 2012 | RAGE | 728 | The rs1800625 polymorphism is potentially useful to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Zeng et al. [69] | 2012 | MD-2 | 726 | The rs11465996 polymorphism is potentially useful to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Zeng et al. [70] | 2012 | LBP | 787 | The rs2232618 polymorphism is potentially useful to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Zeng et al. [67] | 2012 | HMGB1 | 556 | The rs2249825 and the haplotype TCG could be used to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Zhang et al. [71] | 2011 | NLRP3 | 718 | The rs2027432 and rs12048215 polymorphism are potentially useful to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Chen et al. [72] | 2011 | TLR9 | 557 | The rs187084 and rs352162 could be used to estimate the risk of developing multiple organ failure and sepsis in polytrauma patients |
Chen et al. [73] | 2011 | TRL2 | 410 | The rs3804099 and the haplotype ATT could potentially be used to estimate the risk of sepsis and multiple organ failure in polytrauma patents |
Gu et al. [74] | 2011 | IL-4 promoter | 308 | IL-4 -589 T/C polymorphism potentially affects t-helper balance and susceptibility to infection |
Chen et al. [75] | 2010 | TLR4 | 303 | TLR4/2242 polymorphism might be used to estimate the relevant risk and organ dysfunction in polytrauma patients |
Hildebrand et al. [76] | 2009 | CALCA | 137 | Polymorphisms of CALCA had no significant impact in complication development |
Christie et al. [77] | 2008 | MYLK | 237 | Specific polymorphisms found to be associated with the development of acute lung injury |
5.4 Clinical Course and Appropriate Actions
The extent of the inflammatory response is mainly dependent on the magnitude of the traumatic load during injury. This response (SIRS) can be very intense due to the initial injury (first hit) or can be exaggerated from actions and intervention during treatment (second hit) [27, 43]. Any additional interventional (e.g., massive transfusions) or surgical (e.g., prolonged operations, operations with severe tissue damage) load, represents an exogenous hit. Furthermore antigenic load from infections, ischemia/reperfusion injuries, acidosis, respiratory or cardiovascular distress, add an endogenous hit. An uncontrolled inflammatory response may lead to remote organ damage primarily in the lung leading to the development of adult respiratory distress syndrome (ARDS), MODS, and potentially death. At the same time CARS is evolving. If this hypoinflammation state is overwhelming, it may lead to immune-suppression that is responsible for the subsequent septic complications [78, 79]. An uneventful clinical course indicates that a fine balance between these extreme reactions of the immune system has prevailed.
Staging of the physiological status of the patient after the initial assessment and life-saving procedures dictates the sequence and priorities of any further actions. The patient may be classified in one of four categories: stable, borderline, unstable, and extremis [23, 48]. Stable patients have no immediate life-threatening injuries and do not need inotropic support to become hemodynamically stable. Borderline patients have been stabilized during the initial period but the type of their injuries makes them vulnerable to further rapid deterioration. Unstable patients have not achieved the end points of resuscitation and are hemodynamically unstable. Extremis patients usually suffer from the lethal triad and require inotropic support. These patients are very “sick” and usually they succumb as a result of their injuries. Extreme vigilance is required in specific patient groups such as the children, young adults, and athletes since shock can be initially compensated until rapid deterioration occurs. The clinical condition of the patient in any given time reflects a stage in ongoing evolving immune inflammatory reactions. If the magnitude of the initial trauma is well tolerated and physiological markers of stress are not abnormal, early implementation of definite care can be performed with subsequent uneventful recovery. If the initial injury is of great magnitude then hemorrhage control takes priority and temporary stabilization of musculoskeletal injuries utilizing external fixators is performed, in order to minimize the second hit insult and to protect the organism from an exaggerated SIRS, which might lead to ARDS, MODS, or even death. Secondary definitive treatment and reconstruction procedures can be performed when the clinical condition of the patient allows. The rationale behind any intervention is to eliminate the extent of the “second hit” whenever possible [27, 48, 57]. This staged approach minimizes the degree of surgical insult to the patient who is in an unstable equilibrium after major trauma. The management of these patients can be divided into four stages. During the acute phase only the resuscitation and life-saving procedures are performed. After the initial resuscitation and during the primary stabilization period major extremity injuries, arterial injuries and compartment syndromes are managed with DCO. In the secondary period the patient is reassessed constantly and appropriate actions are taken. Major procedures (second hit) are not justified due to the additional burden that may exert to the already compromised patient’s immunological status. Subsequently, between days 5–10 the so-called period of “window of opportunity” definite fracture treatment can be performed [80]. Thereafter, any complex reconstruction procedures can be planned accordingly [6] (Fig. 5.2). Although concerns about longer hospital stay and cost implications have been raised, this approach has definitely modified the perceptions and daily practice of the orthopedic trauma surgeons [81].
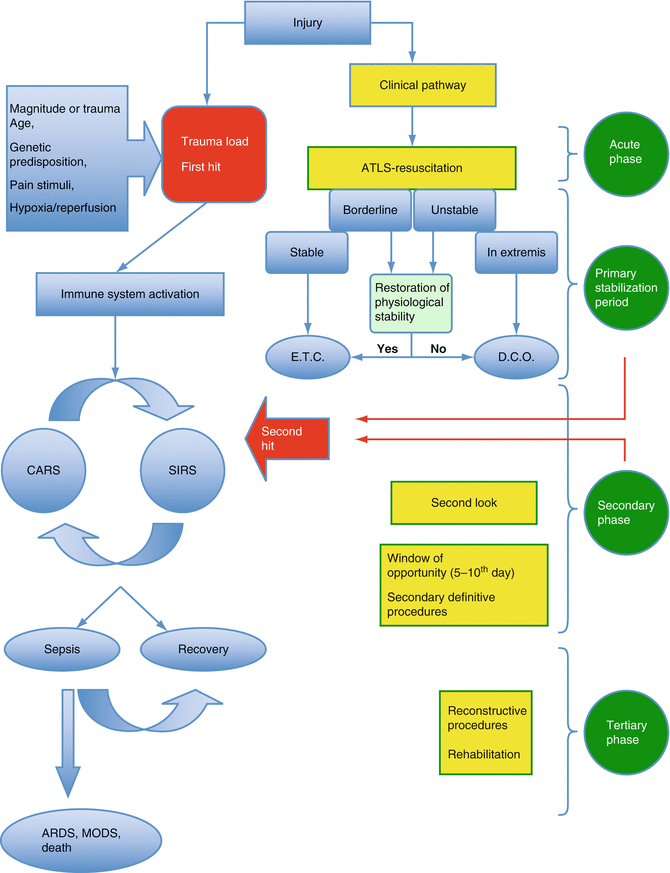

Fig. 5.2
The immune response after trauma, its correlation to clinical status of the patient and the appropriate management in any given phase. ETC early total care, DCO damage control orthopedics, ARDS adult respiratory distress syndrome, MODS multiple organ dysfunction syndrome, ATLS advanced trauma life support, SIRS systemic inflammatory response syndrome, CARS counter anti-inflammatory response syndrome
5.5 Clinical Course and Immunomarkers
From the above described theory of “two” or “multiple hits” is becoming evident that monitoring the patient’s status and clinical course via a scoring system of inflammation would be useful in both guiding our clinical decisions with regard to therapeutic intervention and predicting the possible outcome and complications in the setting of polytrauma. Various attempts have been made and are ongoing to describe the degree of the inflammatory response [82–89].
Immunomonitoring is a term used to describe the value of monitoring the inflammatory markers that are released and can be clinically measured in the setting of polytrauma. The necessity of “immunovigilance” and its possible clinical implications became clearer during the last few years. Until recently we could only draw indirect information regarding the inflammatory status of the patient mainly from clinical markers such as fluid balance [33, 82], lactate and base deficit [90]. However, as our understanding of the complex mechanisms involved in the immune response after trauma has expanded and as our technical ability to measure various molecular mediators has improved a new era in documenting the evolving physiological status of the traumatized patient at the molecular level has been established.
The markers of immune reactivity that may have clinical utility are the acute phase reactants (liposaccharide-binding protein, C-reactive protein, precalcitonin), the markers of mediator activity (TNF-a, IL-1, IL-10, IL-6, IL-8) and the markers of cellular activity (human leukocyte antigen) [91]. While the first category has been proven to be nonspecific for trauma, there is evidence that molecules from the other two categories may have some predictive value.
More specifically TNF-a was one from the first markers that was studied. It has been correlated with poorer outcome in multiple traumatized patients in the intensive care unit but nowadays is not considered a reliable predictive index for the clinical course of inflammation in trauma unless sepsis is present [92]. The clinical utility of IL-1 and IL-10 has not been effectively supported so far [33]. The expression of major histocompatibility complex antigens (MHC class II) at the mononuclear cells of the peripheral blood has been shown to be associated to morbidity due to sepsis after trauma [93]. Many other circulating molecules have been described as potential predictors of the clinical course including the serum amyloid A, procalcitonin, C3 complement, and haptoglobin [94–96]. It appears that a continuously high level or a second rise in their values is correlated with complications and MODS, respectively [82].
Continuous monitoring is more reliable in the case of the pro-inflammatory cytokines and especially in the case of IL-6. The relatively persistent pattern of expression and the long plasma half-life have established IL-6 as the most clinically useful molecule [50]. High values have been correlated to adverse outcome after early surgery [97, 98]. IL-6 is considered to be of prognostic value for systemic inflammatory response, sepsis, and multiple organ failure [82]. IL-6 and SIRS have been correlated to new injury severity score (NISS) and to each other. A numerical value of 200 pg/dL has been proven to be of diagnostic documentation of a SIRS state [50, 99]. In a recent review of the published literature [100] about the clinical implications of Interleukin-6, the positive relationship of the extent of its elevation to the severity/extent of trauma as well as the correlation of its elevation to posttraumatic complications was evident. The authors concluded that further research is needed in order to elucidate the genetic polymorphism related to IL-6 as well as its pathophysiologic role. Large sample population, a sufficient size control group and serial measurements of IL-6 are needed and emphasis in the early posttraumatic period has been recommended. The relatively recent discovery of the alarmins (danger signaling molecules subcategorized as DAMPs and PAMPs) seems to be promising for their use as a predictive marker but up to date there are no powerful studies to support that. On the other hand the characterization and quantification of endothelial injury after trauma has been attempted to be correlated with the inflammatory and clinical status of the traumatized patient. The molecules that are released from the injured endothelium and are measurable in plasma are mainly the selectins (L-, P-, E- selectin), the vascular adhesion molecules, the thrombomodulin and the vW-factor. L-selectin has been shown to be positively related to the prognosis of potential complication after major trauma but definite conclusion cannot be drawn as yet [101].
Finally, the completion of the human genome project has open novel avenues in the clinical setting for the investigation of the genetic make-up of the patient and how this could influence the physiological responses and outcome [102]. Currently there is evidence to support the involvement of various polymorphic variants of genes in determining the posttraumatic course [103]. Single nucleotide polymorphisms result in different immune responses to trauma and might in the future guide an individualized approach to diagnosis and interventions in specific patient groups [63]. Although such an approach appears to be promising, results from different studies have not been reproducible because of the ethnic admixture, variable linkage disequilibrium, and genotype misclassification [103–105]. Further genome-wide and sufficiently powered studies are needed to provide more robust evidence about the contribution of genes in determining the clinical outcome of patients [63]. The need of translation research is also of paramount importance until novel perspectives in the polytrauma pathophysiology find their implementation in clinical practice.
References
1.
Butcher N, Balogh ZJ. The definition of polytrauma: the need for international consensus. Injury. 2009;40 Suppl 4:S12–22.PubMed
2.
Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury. 2006;37 Suppl 5:S3–9.PubMed
3.
Smith RM, Giannoudis PV. Trauma and the immune response. J R Soc Med. 1998;91:417–20.PubMedCentralPubMed

Full access? Get Clinical Tree







