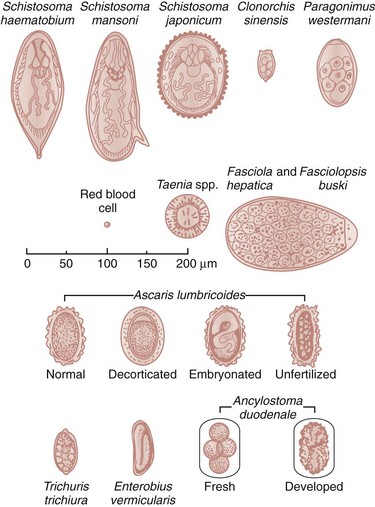
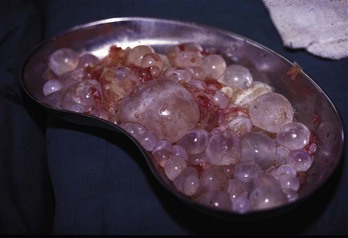
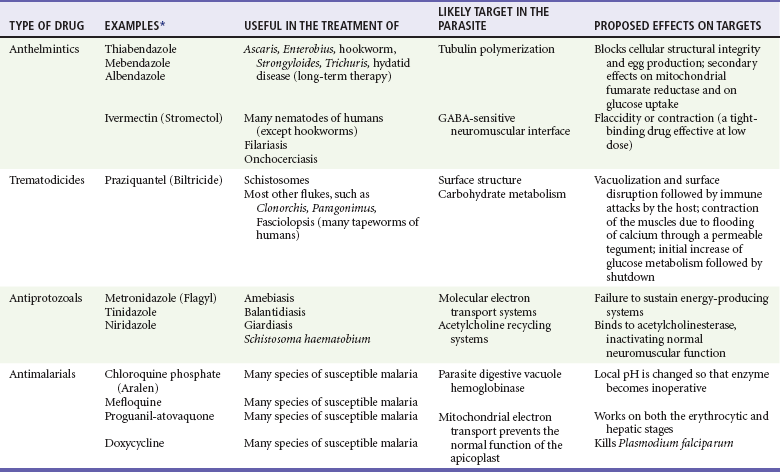
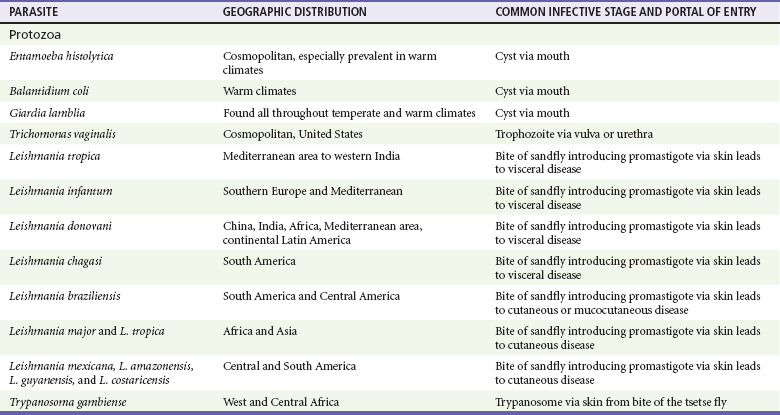
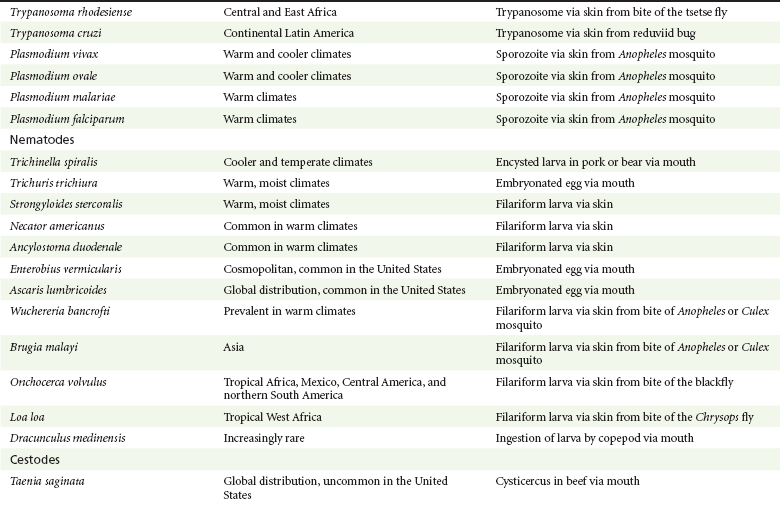
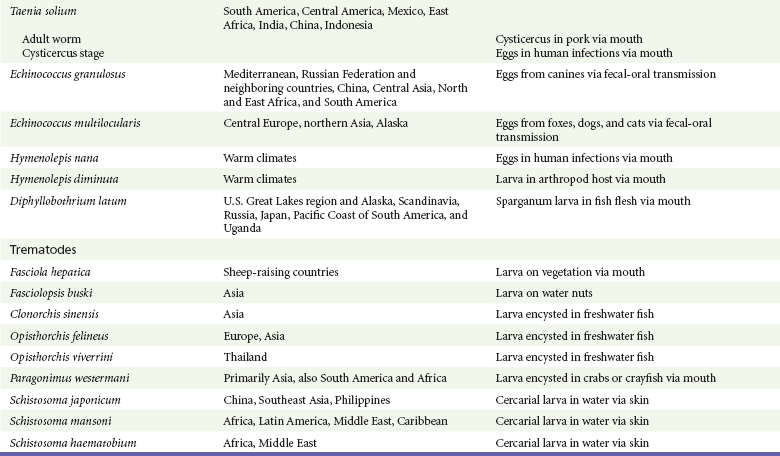
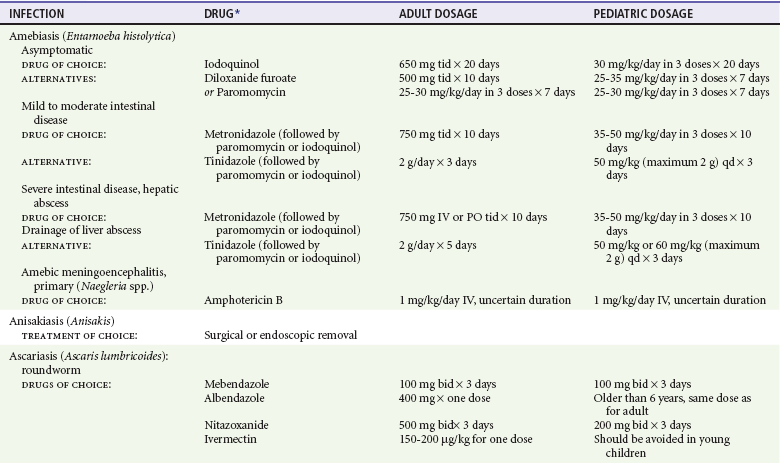
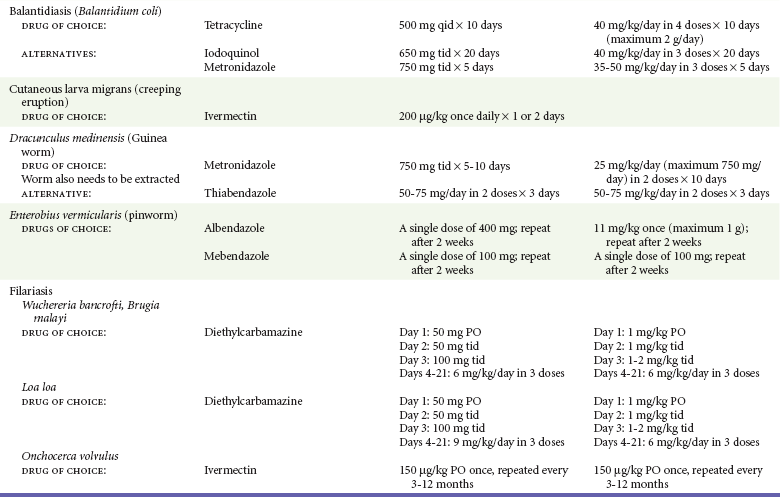
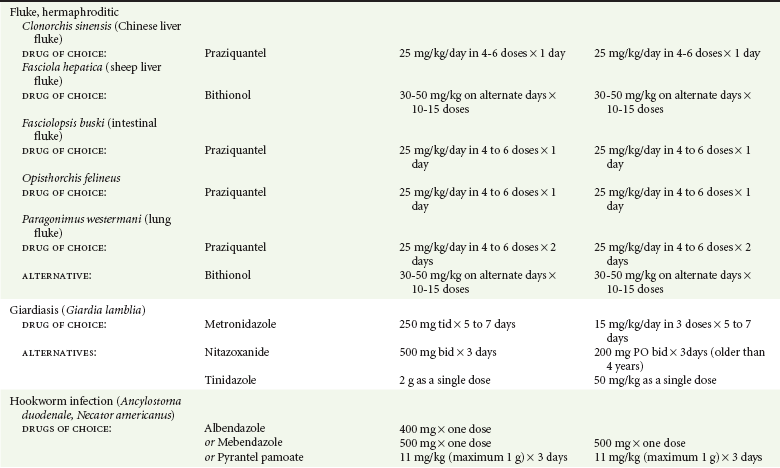
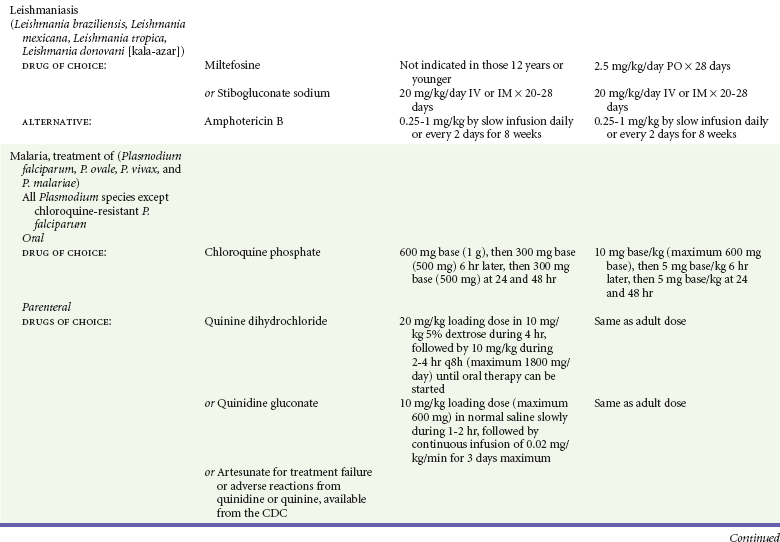
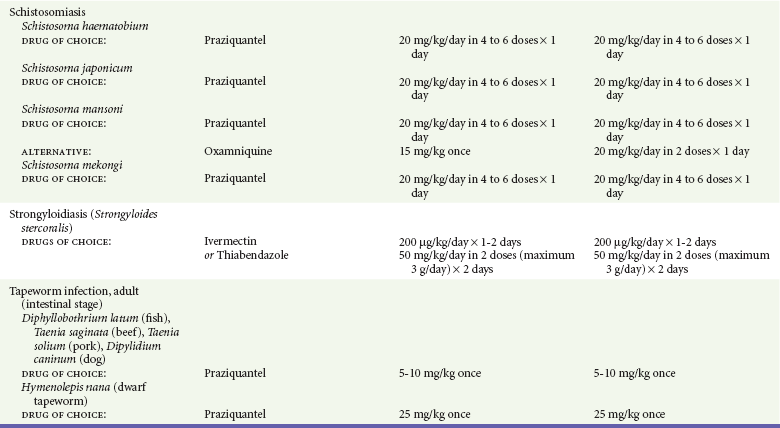
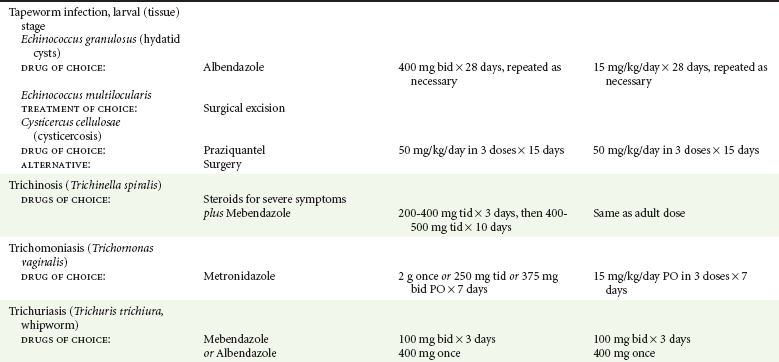
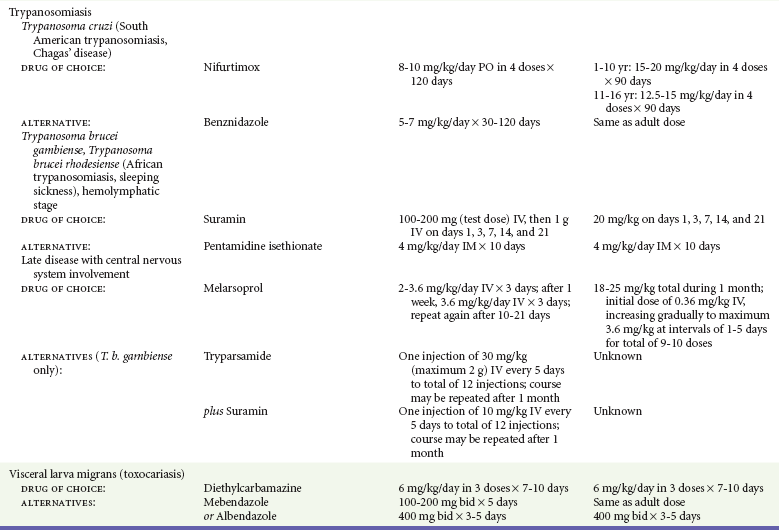
Chapter 133 Parasitology has become increasingly important in the practice of emergency medicine. There has been a dramatic increase in immigration from Southeast Asia, Central and South America, and Africa into the United States during the last few decades. Many of the people in transit have left their countries of origin under dire circumstances, fleeing civil unrest, war, famine, economic hardship, political persecution, and environmental devastation; they often lived in regions where parasitic infections were endemic. Business and adventure travel, including ecotourism, frequently transports immunologically naïve and vulnerable hosts to sites rich in parasitic disease (Fig. 133-1). Patients with human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS) who travel to countries where parasitic illnesses are endemic are at higher risk of contracting these illnesses. Patients with AIDS who immigrate or travel to the United States or Europe may harbor a number of devastating parasitic illnesses. There is a significant prevalence of endemic parasitic disease in many rural areas of the southeastern and southwestern United States and in some parts of Europe. Often, patients with parasitic illness initially seek treatment in the emergency department (ED). Correct diagnosis of and prompt chemotherapy for parasitic illness often lead to rapid recovery (Table 133-1); mismanagement can be disastrous. Osler wrote, “Early in the course of disease, diagnosis is difficult and treatment easy; late in the course, diagnosis is easy and treatment difficult.” Parasitic illness often begins insidiously; without appropriate treatment, the disease will pursue a chronic course, resulting in damage to the host’s end organs, severe morbidity, and even death. To diagnose parasitic infection, the emergency physician must play detective, obtaining a thorough travel history, performing a detailed physical examination, and ordering appropriate laboratory studies. This information must be integrated into a strong understanding of the basic life cycles of parasites, the usual and unusual presentations of infection, and the intersecting geography of the organism and the host. Table 133-1 Drug Classes and Modes of Action of Agents Used for Treatment of Parasitic Disease *Some drugs may be available only from the CDC Drug Service, Centers for Disease Control and Prevention, Atlanta, GA 30333; telephone: 404-639-3670 (nights, weekends, and holidays: 404-639-2888). Parasitic illness should be considered in the differential diagnosis of almost every sign or symptom imaginable, particularly in patients who recently have spent time in areas of the world with endemic parasitic illnesses (Table 133-2). Accordingly, a travel history should be included in the evaluation of most if not all patients presenting to the ED. Some of the most important questions are summarized in Box 133-1. For patients who have recently immigrated to the United States, the history should elicit additional information specific to the country of origin, also summarized in Box 133-1. Table 133-2 Parasites Causing Human Disease: Geographic Location and Portal of Entry Modified from Beaver PC, et al: Clinical Parasitology, ed 9. Philadelphia, Lea & Febiger, 1984. New and more effective antiparasitic agents are continually being developed. The list of drugs used to treat parasitic infestations is large and varied. Table 133-3 includes some of the newest pharmaceutical agents along with other older medications that are still recommended but have become almost obsolete because of toxicity or mediocre efficacy. Table 133-3 Drug Regimens for Treatment of Parasitic Infections *Some drugs may be available only from the CDC Drug Service, Centers for Disease Control and Prevention, Atlanta, GA 30333; telephone: 404-639-3670 (nights, weekends, and holidays: 404-639-2888). Modified from Drugs for parasite infections. Med Lett Drugs Ther 37:99, 1995. Principles of Disease.: The febrile patient with shaking chills and a time-appropriate history of travel to an endemic region requires evaluation for malaria. Plasmodium falciparum, Plasmodium ovale, Plasmodium vivax, and Plasmodium malariae are the species responsible for human malaria. More than 41% of the world’s population lives in malaria-endemic areas (e.g., parts of Africa, Asia, Oceania, Central America, and South America). Approximately 300 million to 500 million clinical infections occur annually, resulting in 1.5 million to 2.7 million deaths.1 Approximately 1500 cases of malaria are diagnosed yearly in the United States. The female Anopheles mosquito is the arthropod vector that transmits malaria after ingestion of gametocytes from infected persons. After sexual reproduction in the gut of the mosquito, sporozoites are released from the salivary glands into the human host during a blood meal. Sporozoites rapidly penetrate the liver parenchymal cells of their host. The protozoans, now termed cryptozoites or exoerythrocytic schizonts, multiply rapidly. Eventual lysis of the hepatic cells results in the release of merozoites into the bloodstream, which invade erythrocytes. In P. vivax and P. ovale infection, dormant hypnozoites can reside in hepatocytes; recrudescence of infections can occur many months to years later. After invading red blood cells (RBCs), the merozoites transform into trophozoites, which feed on the hemoglobin in RBCs. Trophozoites mature into schizonts, which divide asexually into additional merozoites. The RBCs undergo lysis, releasing merozoites into the blood. Although some merozoites are destroyed by the host’s immune system, many enter new erythrocytes. After several repetitions of this erythrocytic cycle, the cyclic process changes, and male microgametocytes or female macrogametocytes may develop instead of merozoites. These gametes subsequently complete the reproductive cycle by fusion, which is accomplished sexually within the gut of a new female Anopheles mosquito after she has taken a blood meal from an infected host. Most people contract malaria after being bitten by an infected vector mosquito in an endemic region. Other mechanisms of transmission have been reported, including blood transfusions, injection drug use with contaminated syringes, maternal-fetal perinatal transmission, transmission from infected organs after transplantation (worsened by immunosuppression), and so-called airport malaria. Airport malaria has been reported in people who have never been in an endemic area but live near or work in an international airport. The infected mosquito is transported from the endemic region and released when the plane arrives at its destination. The mosquito survives long enough to transmit the parasite, although it does not establish itself in the new location.2 Clinical Features.: Most patients with malaria present with cyclic or irregular fevers. Other signs and symptoms include anemia, headache, nausea, chills, lethargy, abdominal pain, and upper respiratory complaints.3 The important difference between P. falciparum and the other malaria species is the capacity of P. falciparum to cause severe organ system damage and death. RBCs infected with P. falciparum are rendered “sticky” and sludge in small arterioles and capillaries, causing ischemia in the host’s metabolically sensitive organs. Acute falciparum infection may have any of the following manifestations: cerebral malaria with cerebral edema and encephalopathy, hypoglycemia (especially in children), metabolic acidosis, severe anemia, renal failure, pulmonary edema, disseminated intravascular coagulation, and death. Diagnostic Strategies.: Light microscopic examination of thick and thin blood films is the “gold standard” approach to diagnosis of malaria. The clinician may have to view several slides and multiple fields to make the diagnosis if the parasite burden is small. Peripheral blood smears are stained with Giemsa or Wright stain and examined with ordinary light microscopy. The diagnosis can be made in a simply equipped laboratory. Even if the parasite is not visualized in the smear, treatment of malaria is indicated if the disease is suspected on clinical grounds. The U.S. Food and Drug Administration has approved the use of an antigen-based rapid diagnostic test for screening of patients. Microscopy should still be performed for all patients who have positive antigen test results to determine species and the severity of parasitemia. Management.: In the past, chloroquine phosphate was the treatment of choice for acute, uncomplicated attacks of malaria. Resistance to chloroquine has been steadily increasing, and the drug is now recommended only in regions of known chloroquine sensitivity: Haiti, Dominican Republic, Central America, and limited regions of the Middle East. For uncomplicated malarial infections in patients from chloroquine-resistant regions, oral quinine and doxycycline given together may be used. Another suitable alternative combination is proguanil-atovaquone. For complicated P. falciparum infection (e.g., cerebral malaria, involvement of multiple organ systems, inability to tolerate oral medication), intravenous quinine (not available in intravenous form in the United States) or quinidine is used. Rapid infusion of intravenous quinine can cause profound hypoglycemia. Patients should not receive intravenous quinine without cardiac monitoring. The artemisinin agents are excellent antimalarials and are available in enteral and parenteral preparations. They have a rapid onset of action and are well tolerated. An oral agent known as artemether-lumefantrine (Coartem) is now available for uncomplicated malaria. The other artemisinins are not approved for use in the United States; however, parenteral artesunate is available as an investigational drug for patients who have complicated malaria not responding to quinidine. To obtain this drug, contact the Centers for Disease Control and Prevention (CDC) Malaria Hotline at 770-488-7788 or, during off hours, at 770-448-7100.4 Primaquine is used to eliminate the hepatic phases of P. ovale and P. vivax to prevent recrudescent disease. Primaquine therapy is contraindicated in patients with glucose-6-phosphate dehydrogenase enzyme deficiency because it will precipitate severe hemolysis. Untreated falciparum malaria can lead to coma and death; early treatment reduces morbidity and mortality. Babesiosis is a malaria-like illness that is becoming increasingly prevalent in the northeastern United States (Babesia microti), the northwestern United States (Babesia gibsoni), and Europe (Babesia divergens). Babesiosis is endemic on Martha’s Vineyard and Nantucket and is suspected, along with ehrlichiosis and Lyme disease, in patients on the cape and islands presenting with the “summer flu.” The organism is a protozoan, similar in structure and life cycle to the plasmodia. It is transmitted by the deer tick Ixodes dammini, the vector of Lyme disease. Several cases have been attributed to transfusions with infected blood.5 Patients with babesiosis experience fatigue, anorexia, malaise, and emotional lability, with myalgia, chills, high spiking fevers, sweats, headache, and dark urine. Other manifestations include hepatosplenomegaly, anemia, thrombocytopenia, leukopenia, elevated liver enzymes (particularly the transaminases), and signs of hemolysis with hyperbilirubinemia and decreased haptoglobin. In an otherwise healthy person, the disease may remit spontaneously. In asplenic, elderly, and immunocompromised patients (especially patients with AIDS and those taking corticosteroids), up to 85% of RBCs may contain organisms. Clinical syndromes in these patients include massive hemolysis, jaundice, renal failure, disseminated intravascular coagulation, hypotension, and adult respiratory distress syndrome. Diagnosis is based on clinical suspicion, multiple thin and thick blood smears (Babesia organisms resemble plasmodia in blood smears), and serologic testing (convalescent titers may not be positive for several weeks after the infection). The treatment of choice consists of quinine plus clindamycin.6 Patients infected with B. divergens tend to be sicker and require more supportive care. Coinfection with Borrelia burgdorferi, the agent of Lyme disease, results in more severe and prolonged illness. Other parasitic illnesses that commonly cause significant fever include schistosomiasis, fascioliasis, African and American trypanosomiasis, leishmaniasis, toxoplasmosis, and amebic liver abscess. Katayama fever may be the initial phase of schistosomiasis. Infected patients report brief exposures to fresh water in endemic areas. Clinical manifestations include spiking fevers, diaphoresis, and cough. Eosinophilia is common.7 Fascioliasis, caused by the liver fluke Fasciola hepatica, is endemic throughout Asia, the former Soviet Union, southern Europe, and South America. Infection begins with ingestion of the metacercariae often found in watercress. Within 6 weeks, patients exhibit right upper quadrant abdominal pain, fever, and eosinophilia.8 American trypanosomiasis (Chagas’ disease) is endemic to Central and South America. The vector, the reduviid bug, sheds trypomastigotes in its feces proximal to the bite site. The host responds to local inflammation and infection by excoriating the site, inoculating the wound with trypomastigotes and initiating systemic spread. Acute Chagas’ disease begins with a chagoma, an infected and swollen bite site, often periorbital, and quickly progresses to fever, malaise, facial swelling, and pedal edema. Parasitization of cardiac muscle leads to the dysrhythmias and ventricular dysfunction that are classically found in late disease (chronic Chagas’ cardiopathy).9 Leishmaniasis is spread to humans by the sandfly and is found in the Middle East, in India, in East Africa, along the Mediterranean coast, and in Brazil. Although leishmaniasis can involve the skin (cutaneous) and the mucosa (mucosal), fever is seen only in visceral leishmaniasis in immunocompetent persons. Signs and symptoms also include massive hepatosplenomegaly, neutropenia, and weight loss.10 The patient who has amebic liver abscesses from Entamoeba histolytica infection presents with high fevers, right upper quadrant pain, and elevated white blood cell count.11 Principles of Disease and Clinical Features.: Cerebral malaria is a common, life-threatening complication of P. falciparum infection. Parasitized RBCs express malarial cell surface glycoproteins called knobs that are sticky. They adhere to capillary walls, causing sludging in the cerebral microvasculature, localized ischemia, capillary leak, and petechial hemorrhages. Clinical manifestations include fever, altered mentation including obtundation, coma, and occasionally seizures. A careful history and early diagnosis and therapy are essential to prevent severe morbidity and death. Management.: Treatment of cerebral malaria consists of intravenous quinine, quinidine, or artemisinin (if it is available); supportive care, including mechanical ventilation for comatose patients and patients with noncardiogenic pulmonary edema; antiepileptics; and correction of acidosis and hypoglycemia (associated with quinine use and cerebral malaria). The mortality rate is high, especially in children, but if the patient recovers, neurologic sequelae are rare. Corticosteroids, including dexamethasone, provide no benefit and can worsen outcome in cerebral malaria. Principles of Disease.: Cysticercosis is caused by the larval form of Taenia solium, a common central nervous system (CNS) pathogen in many tropical areas. Cysticercosis is acquired by humans who eat undercooked pork containing the larval cysts. The adult worm matures in the small intestine; the larval forms may penetrate through the gut wall and end up anywhere in the body. They are trophic for the CNS, muscle, and soft tissue. Clinical Features.: In the brain, the cluster of larvae of T. solium forms an expanding cyst that induces an intense immunologic reaction from the host, including inflammation, fibrosis, and ultimately calcification. Neurologic abnormalities develop when neural tissue cannot accommodate the enlarging cyst. Seizure activity often is the first indication of cysticercosis, which should be considered in the differential diagnosis of new-onset seizures in adults. The diagnosis of T. solium infection is established by the finding of characteristic proglottids (gravid segments) or scolices (worm heads) in stool preparations. Diagnostic Strategies and Management.: Cranial computed tomography (CT) with contrast enhancement or magnetic resonance imaging may reveal an enhancing ring lesion. These lesions can mimic a CNS abscess, metastatic malignant disease, or a primary tumor such as glioblastoma multiforme. Albendazole is the therapeutic agent of choice, and corticosteroids and antiepileptics may be necessary during therapy, particularly if CNS cysts are present.12,13 Neurosurgical consultation should be sought in the treatment of neurocysticercosis because acute obstructive hydrocephalus can occur. Principles of Disease and Clinical Features.: Echinococcus granulosus is another tapeworm capable of causing CNS disease. Cerebral hydatid cysts are loculated structures containing E. granulosus scolices (heads) and the remains of the germinal epithelium, termed hydatid sand. Common types of exposure include ingestion of food or water contaminated by the ova from feces of sheep or cattle infected by the adult worm and close contact with an infected sheep-herding dog that is shedding ova. Infection results in the liberation of the embryo oncosphere into the small intestine. After penetrating the intestinal wall, the larvae travel through the bloodstream to multiple sites for encystment. The liver is the target organ in nearly two thirds of cases, but 7% of patients have brain involvement; infected patients may present with seizures or focal neurologic signs. Diagnostic Strategies and Management.: The diagnosis of hydatid cyst disease is suggested by the appearance and localization of the cyst on ultrasound examination or CT scan. Serologic evaluation of serum or cerebrospinal fluid (CSF) may help confirm the diagnosis. Aspiration of the cyst should not be attempted because of the risk of seeding the host’s body with metastatic cysts. Treatment options include albendazole and surgical resection. Resection of the cyst may cause an anaphylactoid reaction if there is spillage of hydatid sand, which contains parasite antigenic proteins (Figs. 133-2 and 133-3).14
Parasitic Infections
Perspective
Travel History
Principles of Therapy

Presenting Symptoms
Malaria
Babesiosis
Other Parasites Causing Fever
Neurologic Symptoms
Cysticercosis
Echinococcosis
< div class='tao-gold-member'>

Full access? Get Clinical Tree


Parasitic Infections
Only gold members can continue reading. Log In or Register to continue