Chapter 24 Oxygen therapy
In mammals energy is provided by both anaerobic and aerobic respiration, the former being inadequate to provide adequate energy alone, so oxygen is the key ingredient to survival. Aerobic respiration is the most efficient mechanism for adenosine triphosphate (ATP) production. Absence or lack of ATP results in failure of energy-hungry enzyme systems, loss of cell homeostasis, and initially cellular and later organism death. A substantial part of critical care is targeted at treating and/or preventing hypoxia. An understanding of the common pathways that lead to cellular hypoxia from whatever cause is vital to providing appropriate support and treatment to the acutely unwell patient. This chapter will review the pathophysiology of oxygen delivery from atmosphere to cell; methods of assessment; types of therapy that can be acutely administered; and the potential hazards of oxygen use.
PATHOPHYSIOLOGY OF OXYGEN DELIVERY
1 where K is the diffusion constant for a particular gas, A is the surface of a membrane, T is the membrane’s thickness and ΔP the difference in partial pressure across the membrane. About 600 million years ago single-celled organisms evolved into multicellular organisms. As oxygen is poorly soluble in water, diffusion alone became insufficient to deliver oxygen to the cells, and novel methods of delivery evolved, most notably the cardiovascular system.2 This provided the means to deliver oxygen around the body.
OXYGEN DELIVERY
This chain of events is oxygen delivery (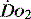 ).
).
STEP 1: CONVECTION – VENTILATION
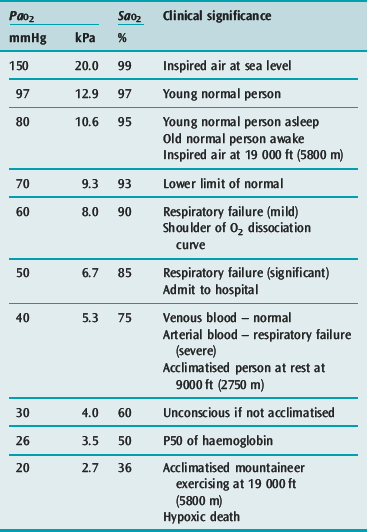
The first step occurs in the lung in the form of pulmonary ventilation. At sea level the partial pressure of oxygen in environmental air is approximately 160 mmHg. On inspiration the air is humidified and mixed with exhaled carbon dioxide (CO2) such that at the alveolus the PaO2 is 100 mmHg. This will vary in different environments and different conditions (Table 24.1). Much of oxygen therapy is based on increasing oxygen delivery into the lungs, whether it be by masks or other devices.
STEP 3: HAEMOGLOBIN BINDING
Oxygen is poorly soluble in water, having a solubility of 0.003 082 g/100 g H2O. Having diffused across the alveolar capillary membrane, the oxygen binds rapidly to the respiratory pigment haemoglobin. The relationship between saturation of haemoglobin with oxygen (SaO2) and PO2 is not linear and forms a sigmoidal shape (Figure 24.1). The P50 is the PaO2 at which 50% of the haemoglobin is saturated. Various factors are known to alter the affinity of haemoglobin for oxygen (Table 24.2). These have teleological advantages as, for example, a low pH or high CO2 at tissue level could imply tissue hypoxia, and the reduction in oxygen-binding affinity, may increase oxygen availability. Similarly, hyperthermia (fever), hypercarbia and an increase in the concentration of 2,3-diphosphoglycerate (2,3-DPG) all move the curve to the right and increase oxygen availability. 2,3-DPG is a byproduct of glycolysis and therefore binds haemoglobin in predominantly hypoxic tissues, facilitating a release of oxygen. Conversely hypocarbia, alkalosis and low concentrations of 2,3-DPG result in a leftward shift of the curve and a higher affinity for binding for any given PO2. Systemic interventions such as alteration in PCO2 or pH will influence the curve and therefore oxygen dissociation and availability.
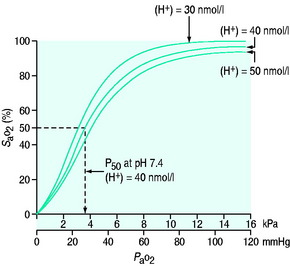
Figure 24.1 Haemoglobin oxygen dissociation curve. Normal curve at 40 nmol/l and shifts to left and right.
Table 24.2 Factors influencing the position of the oxygen dissociation curve
| Factors increasing P50 (curve shifts to the right) | Factors decreasing P50 (curve shifts to the left) |
|---|---|
| Hyperthermia | Hypothermia |
| Decreased pH (acidaemia) | Increased pH (alkalaemia) |
| Increased PCO2 (Bohr effect) | Decreased PCO2 |
| Increased 2,3-DPG | Decreased 2,3-DPG |
| Fetal haemoglobin | |
| Carbon monoxide | |
| Methaemoglobin |
2,3-DPG, 2,3-diphosphoglycerate.
STEP 4: CONVECTION – CARDIOVASCULAR
1 g of haemoglobin binds 1.34 ml of oxygen. Thus changes in the SaO2 and haemoglobin concentration are important in determining the oxygen concentration. Delivery of oxygen is therefore summarised by the formula:
The normal resting 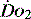 is approximately 1000 ml/min. Oxygen consumption (
is approximately 1000 ml/min. Oxygen consumption (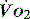 ) at rest is approximately 250 ml/min.
) at rest is approximately 250 ml/min.
where C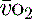 is calculated as (1.34 × [Hb] × S
is calculated as (1.34 × [Hb] × S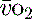 ) + (0.003 × P
) + (0.003 × P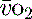 )
)
STEP 5: DIFFUSION – BLOOD TO MITOCHONDRION
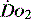 and the rate of cellular uptake and utilisation
and the rate of cellular uptake and utilisationOnce delivered to the cell, oxygen is utilised in the generation of ATP. This is achieved by the production of pyruvate from glucose via glycolysis. This produces only 2 ATP molecules per molecule of glucose. The pyruvate is converted to acetyl coenzyme A and in the mitochondrion enters the citric acid cycle (Krebs cycle), resulting in the production of reducing power to form NADH and FADH2. These molecules are transferred to the electron transport chain and oxidation to water releases the cellular energy currency ATP.3
STEP 6: THE REDOX STATE OF THE CELL
Conventionally oxygen delivery from atmosphere to cell is considered a cascade, implying that a high concentration at one end will cascade down and increase oxygen in the cell. It will increase oxygen available to the cell potentially but the driving force for oxygen to enter the cell and the mitochondrion is the gradient between the partial pressure of oxygen in the cell and outside. Increased oxygen utilisation will reduce that tension and increase the gradient. Conversely, adequate oxygen in the cell will reduce the gradient between the cell and outside and oxygen will not diffuse. The cell determines how much oxygen it uses by creating the gradient that sucks oxygen in – it is not a cascade. Similarly, it is the ATP/adenosine diphosphate (ADP) ratio and probably hydrogen ion concentration that drive ATP production and modify oxygen requirement, i.e. the cell is largely autonomous and uses what it needs.4 While ensuring adequate oxygen delivery is important, excess oxygen is at least theoretically of no benefit.
PATHOLOGY OF OXYGEN DELIVERY (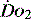 )
)
The level of 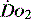 at which
at which 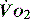 begins to decline has been termed the ‘critical
begins to decline has been termed the ‘critical 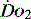 ’ and is approximately 300 ml/min in an adult (Figure 24.2).5 ‘Shock’ is the usual term used in this situation, defined loosely as failure of delivery of oxygen to match the demand of tissue. Commonly this refers to failure of the circulation, but low
’ and is approximately 300 ml/min in an adult (Figure 24.2).5 ‘Shock’ is the usual term used in this situation, defined loosely as failure of delivery of oxygen to match the demand of tissue. Commonly this refers to failure of the circulation, but low 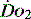 can result from several pathological mechanisms which can occur as a single problem or in combination. Cellular hypoxia can result from each of the stages of oxygen delivery:
can result from several pathological mechanisms which can occur as a single problem or in combination. Cellular hypoxia can result from each of the stages of oxygen delivery:
The above is summarised in Table 24.3 as the types of hypoxia.
| Type of hypoxia | Pathophysiology | Examples |
|---|---|---|
| Hypoxic hypoxia | Reduced supply of oxygen to the body leading to a low arterial oxygen tension | |
| Anaemic hypoxia | The arterial oxygen tension is normal, but the circulating haemoglobin is reduced or functionally impaired | Massive haemorrhage, severe anaemia, carbon monoxide poisoning, methaemoglobinaemia |
| Stagnant hypoxia | Failure of transport of sufficient oxygen due to inadequate circulation | Left ventricular failure, pulmonary embolism, hypovolaemia, hypothermia |
| Histotoxic hypoxia | Impairment of cellular metabolism of oxygen despite adequate delivery | Cyanide poisoning, arsenic poisoning, alcohol intoxication |
The impact of a low 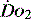 can be made worse by an increase in oxygen demand. Metabolic rate increases with exercise, inflammation, sepsis, pyrexia, thryotoxicosis, shivering. seizures, agitation, anxiety and pain.6 Therapeutic interventions such as adrenergic drugs, e.g. adrenaline (epinephrine)7 and certain feeding strategies can also lead to an increased
can be made worse by an increase in oxygen demand. Metabolic rate increases with exercise, inflammation, sepsis, pyrexia, thryotoxicosis, shivering. seizures, agitation, anxiety and pain.6 Therapeutic interventions such as adrenergic drugs, e.g. adrenaline (epinephrine)7 and certain feeding strategies can also lead to an increased 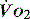 .
.
In critical illness, where oxygen delivery is considered to be in jeopardy, there has been considerable interest in the relationship between 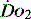 and
and 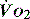 (see Figure 24.2). The presence of signs or markers of tissue hypoxia such as acidosis implies inadequate tissue oxygenation. This could either be from inadequate delivery failing to meet consumption requirements or due to a reduced ability of the tissues to extract oxygen. The former could be corrected by increasing delivery; the latter is more difficult. Historically this has led to the strategy for delivering ‘supranormal’
(see Figure 24.2). The presence of signs or markers of tissue hypoxia such as acidosis implies inadequate tissue oxygenation. This could either be from inadequate delivery failing to meet consumption requirements or due to a reduced ability of the tissues to extract oxygen. The former could be corrected by increasing delivery; the latter is more difficult. Historically this has led to the strategy for delivering ‘supranormal’ 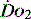 to ensure adequate supply.8,9 There were some intrinsic problems in this approach. It is irrefutable that, if delivery is inadequate, it should be corrected to meet consumption, but much of the early work was based on the relationship between delivery and consumption trying to reach a point where delivery outstripped consumption. As both values are derived from the same root equation and same data, mathematical linkage was inevitable, so as one increased, so would the other.10,11 Also the inotropes used to increase delivery also increased consumption.
to ensure adequate supply.8,9 There were some intrinsic problems in this approach. It is irrefutable that, if delivery is inadequate, it should be corrected to meet consumption, but much of the early work was based on the relationship between delivery and consumption trying to reach a point where delivery outstripped consumption. As both values are derived from the same root equation and same data, mathematical linkage was inevitable, so as one increased, so would the other.10,11 Also the inotropes used to increase delivery also increased consumption.
Clinical studies clearly show that adequate resuscitation to meet oxygen requirements is sensible. In the critically ill going beyond this is not helpful,12,13 although there may be a place for supraoptimal values in the high-risk surgical patient.8,9 In the acute situation the combined use of markers of tissue hypoxia, such as acidosis and lactate, in conjunction with surrogates of oxygen delivery, such as ScvO2, and standard haemodynamic measurements of an adequate circulation have proved beneficial.14,15 So-called early goal-directed therapy is now included in published guidelines for the treatment of severe sepsis (Table 24.4).16 The benefits of this new approach may well have as much to do with the prompt and aggressive improvements in haemodynamics and resuscitation as the values obtained. Timing is probably the significant difference when compared with other applications of the ‘supranormal’ technique. Targetedoxygen delivery is a major debate that is still evolving (Table 24.5). Oxygen delivery can be improved in a variety of ways, from the ambient inspired oxygen through the lungs and cardiovascular system to the cell itself, but once at the cell the ability to manipulate delivery ceases.
Table 24.4 Early goal-directed therapy. A summary of 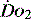 parameters set to achieve in first 6 hours of the diagnosis of severe sepsis with associated hypotension and a plasma lactate concentration of ≥ 4 mmol/l
parameters set to achieve in first 6 hours of the diagnosis of severe sepsis with associated hypotension and a plasma lactate concentration of ≥ 4 mmol/l
| Variable | Parameters |
|---|---|
| Arterial oxygen saturation (SaO2) | ≥ 93% |
| Central venous pressure (CVP) | 8–12 mmHg |
| Mean arterial pressure (MAP) | 65–90 mmHg |
| Urine output (UO) | ≥ 0.5 ml/kg per hour |
Mixed venous oxygen saturations (S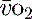 ) or central venous oxygen saturations (ScvO2) ) or central venous oxygen saturations (ScvO2) | ≥ 70% |
| Haematocrit | ≥ 30% |
(After Rivers E et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–77.)
Table 24.5 Summary of targeted oxygen delivery
| Goal-directed therapy |
|---|
| Supraoptimal values in the critically ill – not recommended |
| Perioperative optimisation with supranormal values – possibly useful but controversial |
| Resuscitation against markers of peripheral oxygen use such as ScvO2 and lactate (early goal-directed therapy) – currently advocated |

Full access? Get Clinical Tree


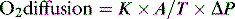
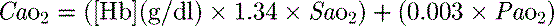
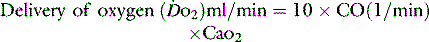
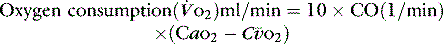
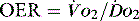
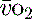 ), which is all the returning blood or central venous blood, can be used as an indicator of global
), which is all the returning blood or central venous blood, can be used as an indicator of global 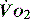 and indirectly the adequacy of
and indirectly the adequacy of 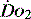 . If oxygen delivery by the microcirculation and cellular oxygen uptake are adequate, then a S
. If oxygen delivery by the microcirculation and cellular oxygen uptake are adequate, then a S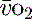 value of 70% usually indicates that global
value of 70% usually indicates that global 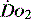 is appropriate. Lower may indicate increased uptake but more often reduced or inadequate delivery. Mixed venous blood is useful for measurement of global
is appropriate. Lower may indicate increased uptake but more often reduced or inadequate delivery. Mixed venous blood is useful for measurement of global 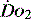 , but it usually requires the presence of a pulmonary artery catheter. The use of central venous saturations has become an alternative surrogate which is usually adequate and considerably more practical.
, but it usually requires the presence of a pulmonary artery catheter. The use of central venous saturations has become an alternative surrogate which is usually adequate and considerably more practical.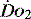 to match changes in
to match changes in 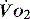 is an adaptation that permits sudden changes in demand such as exercise in which
is an adaptation that permits sudden changes in demand such as exercise in which 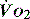 can exceed 1500 ml/min in some cases.
can exceed 1500 ml/min in some cases.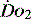 to match
to match 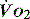 (
(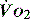 drives the
drives the 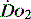 requirement) results in reduction in aerobic metabolism and energy production and necessitates production of ATP by the less efficient glycolytic pathway.
requirement) results in reduction in aerobic metabolism and energy production and necessitates production of ATP by the less efficient glycolytic pathway.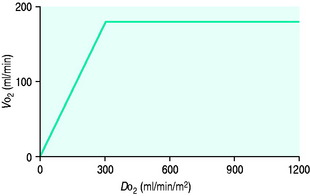
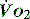 and
and 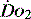 . Above the value of ‘critical
. Above the value of ‘critical 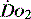 ,
, 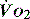 is independent of oxygen delivery. Below this value,
is independent of oxygen delivery. Below this value, 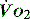 becomes supply-dependent and may be amenable to therapeutic interventions.
becomes supply-dependent and may be amenable to therapeutic interventions.

