HISTORICAL OVERVIEW
Since RA was introduced at the turn of the 20th century, its use has been subject to large waves of enthusiastic and supported practice, followed by periods of declining support, again followed by periods of increased practice. Each change was mainly related to either technical improvements (associated with a decreased rate of complications) or to widely advertised complications, leading to near abandonment of the technique. In 1920, Sherwood-Dunn6 stated that “Since the concentrated solutions of cocaine have been replaced by less toxic agents … death from local or RA has disappeared from surgical practice ….” Thirty years later, Foster Kennedy,7 an eminent New York neurologist, stated that “… paralysis below the waist is too large a price to pay in order that the surgeon should have a fine relaxed field, and that the method should be rigidly reserved for those patients unable to accept a local or general anesthetic.” Although one unfortunate aspect of Dr. Kennedy’s article was the deficiency in anesthetic details, its conclusions were at that time unchallenged.
The Wooley and Roe case, which occurred almost at the same time,8 was generously described in the lay press and led to the near abandonment of RA in the UK and to the supremacy of GA that persisted for many years. In this judgment, it was concluded that permanent paraplegia, which occurred in two patients, resulted from the contamination of the anesthetic solution by the phenol solution in which the ampules were stored for sterilization. By chance, several experts who strongly believed that RA can play a significant role in anesthesia and pain management maintained its use and continued to develop new and better techniques.
A major change occurred at the turn of the 1980s when spinal cord opioid receptors were discovered and led to the development of postoperative analgesic techniques using intrathecal and epidural administration of opioids.9 Rapidly, however, it became clear that epidural and spinal anesthesia were not specific enough to cover every analgesic or anesthetic need, and PNBs were used more widely. The development and wide application of catheter techniques recently contributed to the large application of PNB, improving the outcome of major orthopedic surgery,10 including its use in ambulatory patients who can now leave the hospital earlier with their catheter connected to an analgesic reservoir which distributes the drug for several days, allowing for more stressful surgery being performed in ambulatory conditions.11,12
The Wooley and Roe case also reminds us that the near disappearance of RA was not the result of its poor efficacy but rather as a consequence of high-risk complications. These complications were the consequence of the drugs used which had a low therapeutic index and were also related to needles and catheters that were not sophisticated enough and could by themselves create trauma at the site of puncture. Although the categories of risk that are seen now are similar (drug and technique related), it would be expected that their incidence has decreased significantly due to major improvements in both the pharmacology of drugs and industrial and technical refinements of the equipment. It is, however, not well demonstrated that the incidence and severity of complications related to RA has decreased. This could be related to methodologic bias, as discussed later on in this chapter. It is, however, also possible that one set of complications has decreased through our efforts to improve patient care but that another set of complications has arisen. A part of this may be from new clinical situations (e.g., neuraxial anesthesia and concomitant anticoagulation) and a part may be due to patient factors (i.e., older and less healthy patients).
Early reports described only the complications of spinal and epidural anesthesia and generally stated that RA-induced complications were “rare.”13,14 Coincident with the recent increase in the use of PNBs, three series, including a significant number of PNBs, have now been reported describing the complications, frequency, and outcomes.15–17 A new step has been taken as the relevant question today is to know if modern techniques (such as ultrasound guidance) reduce the risk as compared to traditional ones (such as nerve stimulation or paresthesia seeking). There is a general impression that ultrasound guidance will be a major advance toward reducing the rate of complications (Chapter 17). However, concrete evidence is still lacking for reasons that are mainly related to methodologic factors as described below.
 MEASURING COMPLICATIONS
MEASURING COMPLICATIONS
Our practice of anesthesiology and of RA in particular is not safe, when the rate of complications is compared with the level of safety that has been obtained in some high-reliabilty organizations.18 Reducing the rate of complications and controlling the risks of RA include many well-known strategies such as improved training, use of safer devices and drugs, technologic innovation, and use of quality-improvement programs. Monitoring the rate of complications can be useful but requires a large database, generally including several hospitals, or sometimes needs to be performed at a national level to provide comprehensive results. However, when the overall complication rate is low, traditional methods to assess the level of risk often fail. In a single institution, for example, and even if the volume of procedures is high, the risk of complication is so low that epidemiologic assessment and surveys cannot show if more complications have occurred when comparing two periods of time. When safety has been improved to such a high level that events occur very infrequently, database reporting is no longer efficient. Incident reporting is, by contrast, a useful method to explore the context of a given complication. Organizing a sentinel event system and detecting relevant precursors in near misses are probably the core of the most comprehensive strategy for continuous improvement.19 The rarer the event, the greater the need for in-depth and professional analysis of the few existing cases to capture relevant precursors. At another level, but providing interesting information on a single patient, case reports can be a window on the health care system and journals should facilitate publication of clinical incidents that describe the chain of events and the contributory factors because these reports would have high educational value. Behind the outcome is the process of care and we have to move from “what happened?” to “why did it happen?” Changing the question, however, requires us to change the investigation tools. According to James Reason,20 patent failures are those committed by actors working in direct contact with patients while latent failures represent the consequences of structural, technical, or organizational characteristics often related to management decisions. Root-cause analysis used by the Joint Commission in the US21 or the systems analysis used by Vincent et al.22 in London are typical examples of innovative methods to study the system errors. It is obviously an added value to share not only the result (i.e., the incidence) but also the very content of the case analysis with a large number of practitioners. Because the level of risk associated with RA can be considered either high or low depending on the comparator used, both traditional and innovative strategies of risk control should be implemented (Chapter 1).
 REPORTING COMPLICATIONS
REPORTING COMPLICATIONS
Complications are frequently not reported or reported inaccurately. This stems from a variety of factors that are described below (Box 2-1).
BOX 2-1 Difficulties Measuring and Reporting Medical Complications
The frequency of unusual or rare complications is difficult to measure. Individual case reports lack a denominator, whereas large studies of adequate numbers of subjects are difficult to perform.
The reporting of medical complications can be problematic. For example:
 The anesthesiologists providing care may or may not be expert in the techniques being studied.
The anesthesiologists providing care may or may not be expert in the techniques being studied.
 How the complication is reported may be influenced by the nature of the study. For instance, a voluntary reporting of complications versus controlled reporting in a randomized clinical trial.
How the complication is reported may be influenced by the nature of the study. For instance, a voluntary reporting of complications versus controlled reporting in a randomized clinical trial.
 The timeframe under which the study is conducted may not be suitable to identify all complications.
The timeframe under which the study is conducted may not be suitable to identify all complications.
 Publication bias may be present on the part of journal editors. For instance, there may be a reluctance to publish a single case report of a previously described complication.
Publication bias may be present on the part of journal editors. For instance, there may be a reluctance to publish a single case report of a previously described complication.
Practitioner Bias
It is basically difficult to study rare events, and RA-induced complications belong to this category. The incidence can be estimated through surveys and large series. Well-performed studies including sufficient numbers of cases are rare. Moreover, most studies come from institutions where RA is well accepted and where physicians who perform the blocks are highly trained, and this may not reflect the true rate of complications. We indeed now know that training is associated with increased performance and a reduced rate of complications. In the rare large-scale studies reporting data from both specialized and nonspecialized centers (i.e., where physicians perform a high or low number of procedures), the incidence of complications appears to be much greater than in high-volume institutions. In a study reporting neuraxial anesthesia-induced infectious epidural abscess from all institutions in Denmark (i.e., including both high and low-volume centers and thus including a nonselected population of physicians), the incidence was much higher than in other studies.23
Reporting Bias
Uncertainty regarding the true incidence may also arise from the quality of data reporting: incidence may increase as a result of better reporting or a better collecting method. In two classic studies, each assessing a large number of spinal blocks, Dripps and Vandam13 assessed the risk associated with the use of procaine and tetracaine in 10,098 patients, while Phillips et al.14 monitored 10,440 patients after lidocaine spinal anesthesia. The incidence of complications was monitored prospectively by directly questioning all patients on the day after surgery. A zero incidence of severe complications was described, and these studies started an optimistic period during which the development of RA was rapid as the technique was perceived to be safer than general anesthesia. In one of these studies, the quality of postoperative monitoring and patients’ interviews was so good that the authors were able to describe “complaints confined in lumbar and sacral areas of the body which generally lasted few days” and the complaints were described as “numbness, tingling, heaviness or burning and of minor significance in the lives of the individuals affected.”24 The authors had probably described what we now call transient neurologic symptoms (TNS), but these symptoms were dismissed probably because these complications were so minor that they were felt to be clinically insignificant. Although such a prospective design should have guaranteed an excellent quality of data reporting, only a few questions were asked to each patient, that is, “have you had any problems related to the anesthesia…” and “would you recommend spinal anesthesia”24 which is obviously too simple to ensure adequate reporting. Obstetric patients were the core of these studies, and we now know that the incidence of some complications (i.e., hearing loss and TNS) may be different in this category of patients.25,26
Reporting bias can also occur because of the retrospective nature of many past large surveys or absence of detail. Fine analysis of individual cases can often only be found in prospectively reported cases or in cases that have been associated with litigation. In the first survey performed in France,16 cases were counted prospectively, but details of complications were collected at the end of the 6-month period of the study. It appeared that the information was not as accurate as expected initially. In the American Society of Anesthesiologists Closed Claims project, detailed description of cases was also available as they were extracted from insurance claim files which typically include narrative statements from the personnel involved, medical records, expert and peer reviews, deposition summaries, outcome reports, and the cost of settlement or jury award.27
Timing Bias
Moreover, as some complications become apparent only several days after the block, a questionnaire study based on a single interview performed the day after surgery may have missed some complications. The timing at which complications become apparent is indeed variable. Although some patients complain of paresthesia, pain, or motor disturbances within hours after surgery, in other cases, the neurologic complications may only become apparent after several days. This is obviously the case after postoperative continuous infusion of local anesthetic, which precludes any neurologic evaluation before the block has worn off. In this situation, although continuous infusion has advantages, namely absence of any pain during the first days, it does also lead to complete anesthesia and sometimes complete motor block during the same period of time, precluding any neurologic assessment. This suggests that analgesic techniques which allow for some partial recovery at (regular) intervals (i.e., catheter techniques linked to a patient-controlled analgesia (PCA) device or intermittent bolus infusion) may have advantages over the continuous infusion of local anesthetic drugs alone. In patients receiving epidural analgesia, addition of an opioid clearly reduces the dose of local anesthetic needed and thus the motor block, thereby facilitating monitoring of the block. Regular and adequate monitoring and early diagnosis of neurologic complications is important as early detection may allow for rapid recovery (and sometimes lead to early therapeutic actions) while late discovery of a complication may lead to definitive neurologic sequelae. In our experience with SOS-Regional Anesthesia (SOS-RA) Hotline Service over the past 12 years, we have often seen neurologic complications arising in institutions where anesthesiologists were highly trained and where surgeons had a high confidence in RA but where monitoring and nurse training were not adequately organized to allow for rapid diagnosis of complications. Physicians do place a greater emphasis on performing the block than on organizing the postoperative surveillance.
Publication Bias
Large series have taught us much about RA-induced complications, but case reports also have largely contributed to our knowledge. However, even large series may not accurately report rare complications. Looking at the SOS-RA series,17 one may believe that neuraxial blocks are not associated with a risk of major neurologic event (as none occurred during the 6-month period of the study), while it is obvious that this complication can occur and is a major threat for patients.
Although they are often considered as being minor scientific contributions, case reports have sometimes had a greater impact on our clinical practice than most randomized trials. Albright’s description of a small series of cardiac deaths after bupivacaine administration,28 Schneider et al.’s29 description of TNSs after lidocaine spinal administration, cases of cardiac arrests following large dose of ropivacaine,30,31 and the recovery after cardiac arrest following bupivcacaine toxicity using “lipid rescue”32 are only four examples of how case reports can strongly impact on the thinking of a whole medical specialty.
One should also consider the tendency of journals to accept a first or second case report of a rare complication, but then not accept subsequent reports. However, since the complication is rare, the next step often cannot be taken (i.e., reporting large case series or doing randomized controlled trials).
 PREVENTING COMPLICATIONS
PREVENTING COMPLICATIONS
Society’s View
Society’s expectations of complications are frequently at odds with the medicine’s view of complications (Fig. 2-1). Society may be much more likely to expect perfection from physicians who realize that perfection is a worthy ideal which may rarely be attainable. A zero complication rate is not even attained in high-reliability organizations that are considered ultra-safe because they are associated with a 1/10,000,000 rate of complications. “Six-sigma” strategies that have proven their efficacy in the industrialized world are being implemented in medicine today with the hope that they will help reaching this ultra-low level of danger, but initial data in patient-related processes do not show as excellent results as expected.33
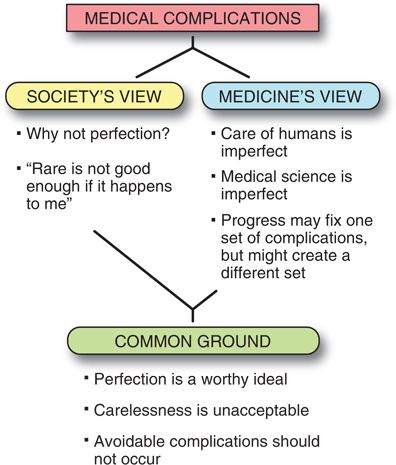
FIGURE 2-1. Medical complications: the conflict between society’s view of risk and medicine’s view of risk.
Many complications that physicians consider minor because they do not lead to long-term sequelae (such as hoarseness after an interscalene block, or a meningeal puncture headache that resolves) are considered major complications to those who suffer from them. Such complaints mainly occur in minor procedures where patients’ expectations of complete and rapid recovery are not met or in situations in which the anesthesiologist has convinced the patient that regional anestheia is a better choice than general anesthesia.
Medicine’s View
Scientific Limitations to Understanding Complications: The Meaning of Paresthesia
It is only recently that series including a significant number of PNBs have been reported to describe their complications, frequency, and outcome.15–17 These reports came from European institutions where the use of nerve stimulation was already accepted and had introduced a relatively new debate relating to the significance of paresthesia occurring during puncture; some experts searching deliberately for a paresthesia to block the nerve while others felt that a paresthesia is associated with an increased rate of complications. This debate is far from being closed as contradictory information exists. Complications apparently related to the regional block can occur even though a gentle technique has been applied and no paresthesia has occurred, while by contrast it has been shown that immediately after a paresthesia has occurred, nerve stimulation can fail, suggesting that the nerve is not as close to the needle as expected or that paresthesia may have different meanings.34–36 Experts using ultrasound guidance have also recently reported several cases in which the needle makes physical contact with a nerve, but no paresthesia is felt by the patient.
Diagnosing and Treating Complications
One significant problem is the difficulty attributing a given complication to RA (i.e., to determine if RA is the cause of the neurologic injury) when surgery, positioning, or a preexisting disease might have caused the complication. Two clinically significant and frequent situations are obstetrical nerve injuries and neurologic complications after hip replacement. RA is often blamed first, whereas the relative incidences of complications related to the procedure versus to RA should lead us to first blame the delivery or surgery and not RA. Postpartum nerve injuries occur 5- to 10-fold more often as the direct consequence of vaginal delivery than as caused by RA.37–39 Vaginal birth of a large newborn after a long labor and using instrumental delivery are traditional risk factors, but these situations often accompany highly painful labor for which epidural analgesia will be requested more often by the parturient.40
A similar situation is seen after hip surgery in which the intrinsic rate of sciatic nerve injury (i.e., in the absence of any other contributory factor such as PNB) ranges between 0.5% and 3%.41,42 However, RA is often blamed by surgeons and patients, and anesthesiologists need to make major efforts to correct the diagnosis. As for obstetric nerve palsies, surgery itself causes nerve injury much more often than RA, and this should be emphasized. Female patients and patients operated for hip dysplasia appear to have a higher risk of neurologic injury. Also, the risk of neurologic injury following total hip arthroplasty appears to be higher with revisions/reoperations and with an inexperienced surgeon or with a misplaced retractor. Nerve injury can also occur postoperatively and be caused by cement migration. To prevent the occurrence of intraoperative nerve injury, somatosensory evoked potentials have been used, but the efficacy of this technique has not been demonstrated in total hip arthroplasty. Other experts have also chosen to avoid using sciatic nerve block in patients at high risk of postoperative nerve injury to minimize conflicts and litigation. Note also that sciatic nerve block may interfere with intraoperative neurologic monitoring. We agree with Ben-David et al.43 that “with the expansion of regional anesthetic techniques in acute pain management, the finding of a new postoperative deficit must be jointly investigated by both anesthesiologists and surgeons. Timely and open communication between services is critical because rapid intervention may be essential to achieving full recovery of an affected nerve.” Adequate follow-up and evaluation using a systematic method involving experts is extremely important when deciding if the complication is or is not related to RA. Early examination by a neurologist is extremely useful to precisely define the clinical picture and help localize the nerve lesion. The neurologist is not asked to make any firm assumption on the mechanism of the nerve injury. In many situations, it is recommended to perform ultrasound or computed tomography scanning to search for an hematoma or migrated cement. This can lead to early reoperation and rapid recovery of the nerve injury.
Electrodiagnostic techniques are extremely useful, and the first examination should be done as early as possible (within the first 48 hours) because abnormal findings at this stage strongly support the role of a preexisting neurologic disease. They will be repeated within 3 to 4 weeks where more definitive information can be obtained about the site, nature, and severity, all factors that can guide prognostication.44 Electromyography provides findings suggestive of denervation (i.e., fibrillation potentials and abnormal muscle unit recruitment) while nerve conduction studies provide an estimate of the number of axon loss by analysis of the size of the muscle response. Of crucial importance, it provides some information on the site of the injury which is often the cause of debate between the surgeon and the anesthesiologist. Separating the role of RA and surgery respectively is sometimes easy (e.g., sciatic injury at the popliteal level during knee replacement in a patient who has undergone femoral and sciatic block at the hip), but this is not always the case when the block and the surgical location are situated in the same nerve segment. In this latter case, indirect arguments are useful, such as relative risk of injury and patient history. In our experience, anesthesiologists often need some help, and this is a daily role for SOS-RA experts to provide some guidance on the diagnostic tools to be used and their timing, but also to discuss arguments that can help in separating the role of RA from other causes (surgery, positioning, and patient history). Whatever the cause, anesthesiologists are also often asked to follow these patients and manage neuropathic pain, which can be difficult to treat, and advice on management is often provided.
Training Issues
In experienced hands, the complication rate is logically lower than when trainees are studied. This emphasizes the role of adequate supervision during the training period and the methods to ensure a rapid learning curve. Although it is out of the scope of this chapter to overview all aspects of safe, efficacious, and ethical training, RA is, as surgery, a field in which the period of learning is critical. It is still too often that physicians try for the first time in their next patient a new block technique that was described (as being easy to do, safe, and with a high rate of success) by an enthusiastic speaker in a meeting. Excellent knowledge of anatomy is critical and should be learned precisely before puncturing any patient. Simulators now exist (manikins or computer programs), which may play a key role in rapid training. Although studies show that a mean number of blocks (often found to be around 50) is necessary to ensure adequate training,45 every trainee should be followed individually as the learning curve is likely to be different, some being rapidly proficient in the technique while others need more time to reach a minimum success rate. It should be noted also that the minimal number of blocks performed does not guarantee a 100% success rate or avoid the occurrence of complications, thus requiring the trainee to maintain his/her vigilance and perform the blocks with gentleness and attention to continue learning from each new experience.
 TRENDS IN RA COMPLICATIONS
TRENDS IN RA COMPLICATIONS
Major versus Minor Complications
It is also difficult to compare the early studies and the more recent ones as definitions of complications vary and preclude any good comparison. While some studies aim at reporting all complications and thus provide an overview of the risks associated with RA, others have chosen to report only major complications to avoid difficulties in analysis and to facilitate the understanding of the topic. Cardiac arrest has a clearer definition than “minor” neurologic complication for which the threshold line between major and minor is more difficult to draw. Moreover, many minor complications are only transient and have only a small impact on patients’ lives. Major complications are however less frequent and may thus be difficult to study. By contrast, minor complications may be more common and may be surrogate endpoints that can lead to an interesting analysis while being easier to study. Moreover, minor complications are easier to discuss because they carry less emotional weight as the patient outcome is not endangered. In voluntary incident reporting systems, the risk of underreporting is reduced with minor complications because these incidents do not lead to negative comments regarding competence and are easy to discuss between peers. By contrast, minor complications that are by nature less important may easily be omitted in these voluntary reporting systems. Although reporting only major complications such as cardiac arrest should make it easy to compare incidences between studies (and thus show whether or not the risk associated with RA has decreased), other factors increase the complexity.
Systemic Local Anesthetic Toxicity
Systemic local anesthetic toxicity during RA can lead to death. Apart from early implementation of the traditional test dose, consistent efforts have been made to reduce this risk (Chapter 7). The commercial release of drugs with lower cardiac toxicity (ropivacaine and levobupivacaine) has certainly played a role. Indeed, in the Auroy et al.16 survey describing data obtained in 1998 to 1999, the authors suggested that they had observed a decreased rate of local anesthetic-induced systemic toxicity when compared to their previous survey. The beneficial effect of these drugs has been demonstrated in many preclinical studies, but case reports appearing increasingly in the clinical literature have confirmed a less toxic profile. What seems for example a major safety advance is the fact that almost all ropivacaine-induced cardiac arrests are easily resuscitated, and to our knowledge ropivacaine induced-death is an extremely rare event.46 Levobupivacaine can sometimes cause convulsions or cardiac arrest, but to our knowledge, in no case has the event has been terminated by patient death.
Another significant advance has been the introduction of the “lipid rescue” technique. From the pioneering experimental work of Weinberg,47 we have learned that using an infusion of a lipid emulsion, the life-threatening events associated with bupivacaine infusion can be reversed. More recently, several case reports have described the reversal of cardiac arrhythmias and neurologic involvement both in adults and children. We however only rely on case reports since clinical studies cannot be performed in this context. Although at least one case report has described the failure of lipid rescue, most published cases describe an impressive effect with almost immediate reversal of toxicity.
Even practitioners with limited experience have rapidly discovered that using ultrasound guidance, nerves can be easily surrounded with only a very small amount of the local anesthetic solution. Studies have rapidly confirmed this notion. Marhofer et al.48 have indeed shown that a fourfold reduction in the amount of local anesthetic can provide an effective axillary plexus block (from 0.4 to 0.11 mL/mm2 cross-sectional area). The trend toward reduced doses has culminated with the work of O’Donnell and Iohom49 who have reported a successful axillary brachial plexus block with as little as 1 mL of 2% lidocaine per nerve. Even if these volumes reflect these authors’ extraordinary expertise, we now confidently use smaller volumes in our everyday practice. As the total dose administered is one major cause of systemic toxicity, ultrasound guidance is expected to decrease the rate of cardiac and neurologic complications due to high plasma concentrations. Cases of local anesthetic systemic toxicity continue, however, to be reported in patients in whom the block was performed using ultrasound, suggesting that this technique may not completely protect against the occurrence of this complication (Chapter 17). From the data available today, the incidence of complications related to systemic toxicity has not yet decreased consistently. This may reflect that all team members are not still perfectly trained and that increasing experience will decrease the ratio. Visualization of the needle tip is not always easy during an ultrasound-guided procedure and vessel puncture remains possible, thus leading to direct intravascular injection and signs of toxicity.
Cardiac Arrest
We discuss in this chapter only cardiac arrest caused by hemodynamic disturbances, as systemic toxicity of local anesthetics has been reviewed above. When looking at studies describing cardiac arrest during the last 50 years, no significant decrease in the incidence of this complication can really be observed. Old studies reported cardiac arrest and death as a rare complication of spinal anesthesia (1/10,440)13 (0.3/10,000),50 while in 1995, Scott and Tunstall51 reported two cardiac arrests in 122,989 obstetric patients who had received epidural or spinal anesthesia, that is a very low incidence as well. These results should be compared with those obtained for spinal anesthesia-induced cardiac arrests in nonobstetric studies. Auroy et al.17 showed that spinal anesthesia was associated with a 2.7/10,000 rate of cardiac arrest in nonobstetric patients, a much higher incidence than in the studies mentioned above. Obstetric patients are overall young and healthy and have a lower risk of complications than other patients who are receiving RA. In the Auroy et al.’s16,17 study, the authors demonstrated that patients who died from cardiac arrest after spinal anesthesia were much older, had an increased ASA score, and underwent hip surgery more often than those who survived.
Neurologic Injury after Neuraxial Blocks
Spinal hematoma after neuraxial blocks are more frequent after orthopedic surgery than after obstetric anesthesia, and this can be explained by the role of thromboprophylaxis and by enlarged osteoporotic vertebrae which narrow the spinal canal and increase the risk that a small hematoma causes a clinically significant complication46 (Chapter 4). In their review of neurologic complications after neuraxial blocks in Sweden, Moen et al.52 emphasized the role of spinal stenosis, which was associated with an increase in the incidence of spinal hematoma and cauda equina syndrome. Since this problem was highlighted by Moen et al., several recent reports have confirmed the role spinal stenosis may play as aggravating the risk of neurologic complications after neuraxial anesthesia.53,54
Spinal hematoma has been largely related to the use of anticoagulants. In his excellent 1981 review, Kane55 emphasized the role of anticoagulants in the occurrence of this complication. In the 1980s, the drug most widely used was unfractionated heparin. We have progressively learned how to use unfractionated heparin in the context of RA, and we have succeeded in mastering the rate of complications associated with it. Disappointingly, an epidemic of spinal hematoma following the use of high prophylactic doses of low-molecular-weight heparin occurred in the US nearly 20 years later,56 mainly associated with an excessive dosage and uncontrolled timing of injections. Analysis of these cases led to new guidelines emphasizing a more restrictive approach,57 and the current feeling is that the risk of low-molecular-weight heparin–associated spinal hematoma is well controlled. Should we however be frightened by the massive arrival in our patients’ prescriptions of antiplatelet therapy (often prescribed as dual treatment)? Although the present recommendations are wise, it remains possible that complications may increase since a recent report suggests that RA may be safely performed in patients receiving clopidogrel.58 In a slightly different context, a recent review article analyzing the risk of spinal hematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals states that based on the current litterature, a platelet count of 80,000/L is a “safe” count for placing an epidural or spinal anesthetic and 40,000/L is a “safe” count for lumbar puncture.59 They further state that “For patients with platelet counts of 50,000–80,000/L requiring epidural or spinal anesthesia and patients with a platelet count 20,000–40,000/L requiring a lumbar puncture, an individual decision based on assessment of risks and benefits should be made.” Although these investigators may be right, such statements may lead to more liberal practice, and there is a strong need to monitor closely what happens with these new guidelines to see if the complication rate rises again.
At the other end of the spectrum, by contrast, TNS are a clinical situation for which our understanding has rapidly increased through a combination of clinical and experimental studies which have precisely defined the incidence and the potential mechanisms including the role of drugs, namely lidocaine. Our knowledge on lidocaine-induced neurotoxicity has increased very rapidly and is such now that evidence exists that apoptosis is the main effect through which lidocaine may produce direct nerve injury.60 Studies are also underway to demonstrate the benificial effect of mitogen-activated protein kinase inhibitors to reduce the cytotoxic effects of lidocaine.61
Neurologic Complications after PNB
Suggested etiologies include mechanical trauma from the needle, nerve edema and/or hematoma, pressure effects of the local anesthetic injectate, and neurotoxicity of the injected compounds, both local anesthetics and adjuvants (e.g., epinephrine) (Chapters 8 and 14). A renewed interest in these complications has arisen because of the increased use of these techniques and the improved knowledge that has been gained by the use of ultrasound technique. Sonography has indeed highlighted the frequency with which the needle tip penetrates in the nerve and produces intraneural injection (identified by nerve swelling). We have also learned that despite this high frequency, related complications are rare, leading to the idea that only intraneural, intrafascicular injection is dangerous. Robards et al.62 reported that in 83% of patients, a motor response (using current intensity of 0.2 to 0.5 mA) could only be obtained on the entry of the needle into the nerve. Again, despite the high frequency of intraneural injection, no patient developed postoperative neurologic dysfunction.
These studies are extremely useful as they suggest some preventive measures: the minimal current intensity should probably not be diminished below 0.5 mA, injection should be stopped (and the needle slightly withdrawn) when nerve swelling occurs, and injection pressure should be controlled. These precautions are aimed at avoiding any form of intraneural injection. Because intraneural extrafascicular injection, however, seems safe and is often associated with a more rapid onset of the block, some authors suggest that intraepineural injection should be evaluated as a worthy replacement of the traditional extraneural technique63 (Chapter 17).
Although ultrasound guidance has helped us to better understand anatomy and procedure-related complications, epidemiologic data do not yet show a clear benefit as the largest studies today do not suggest a reduced rate of neurologic complications. Brull et al.64 have published a nice study which can be considered as providing an estimate of the neurologic risk before the introduction of ultrasound guidance. When comparing the risk provided by Brull et al., the studies by Fredrickson et al.65 and by Barrington et al.66 do not show a convincing reduction. The reason why the difference is not obvious is unclear. It might be that contrary to our thoughts, ultrasound really has no effect on the risk level. Alternatively, this may be because of lack of statistical power or because physicians were not completely trained at the time these studies were performed. It is our premise that ultrasound will reduce the rate of severe neurologic injury as some surrogate endpoints that are easier to evaluate are already different. Liu et al.,67 for example, has indeed shown that ultrasound reduces the number of needle passes needed to perform interscalene block as compared to a nerve stimulation technique.
Continuous Peripheral Catheters
As techniques of PNB improve and their indications increase, the need to use catheter techniques also becomes obvious to prolong the duration of analgesia. Capdevila et al.68 have reported a series of 1,416 PNBs with a catheter maintained for postoperative analgesia during 2 to 3 days. Although they described only three cases of neurologic complication (which all resolved within weeks or months), the incidence was 0.21%. In a similar study performed on 405 axillary catheters used for postoperative analgesia, new neurologic complications occurred at a rate of 0.5%, and the authors concluded that the risk is similar to that of single-shot techniques.69 These incidence figures are not, however, low and require attention and future studies. The apparently high incidence of complications in this setting may be related to various factors. Although new local anesthetics are inherently safer than older ones, prolonged contact with the nerve sheath may be dangerous. Our knowledge and optimization of catheter use are becoming progressively refined. Stimulating catheters provide an advantage compared with nonstimulating catheters at various block locations as a recent semiquantitative systematic review of 11 randomized studies concluded that there is evidence of improved efficacy as measured by local anesthetic volume required, rescue analgesics, and complete surgical block compared with nonstimulating catheters.70 Ilfeld et al.71 compared administration of ropivacaine for 24 or 96 hours after knee arthroplasty and concluded that a 4-day ambulatory continuous femoral nerve block was beneficial as regards to both discharge criteria and pain relief. These studies are useful as they help define better how to use these perineural catheters, and by optimizing their use the incidence of complications might be reduced. Technique-related characteristics also need to be explored as trauma from the catheter may also lead to neurologic complication. Mariano et al.72 recently showed that for popliteal-sciatic perineural catheters, ultrasound guidance takes less time and results in fewer placement failures compared with stimulating catheters, suggesting a potentially reduced risk of complications.
Another interesting lesson from the work by Capdevila et al.68 is that catheter cultures were positive in 29% of cases, inflammatory local signs were seen in 3% of their cases, and one patient developed a severe psoas abscess. Although infectious complications can occur with single-shot PNB techniques, it seems obvious that perineural catheter techniques are more prone to be associated with infection. Diabetic patients are highly sensitive to infection, and Staphylococcus aureus was found in three of the four cases published. Local inflammatory signs were frequent. These data suggest that excellent antiseptic preparation is mandatory, and catheter management should be as rigorous as with central venous catheters.
New Concerns
In the previous paragraphs, we have presented a overall optimistic view supported by many articles which show that novel techniques, drugs, and practices do have a positive effect on minimizing the rate of RA-induced complications. While this is the major tendency, some alarm signals suggest that we have not completely defined the scope of the question and that unknown (or poorly identified complications) can represent a significant threat to our patients and require greater attention in the near future.
Local anesthetic induced chondrolysis is one example of a complication that was virtually unknown 5 years ago. With the development of “pain pumps” which provide direct intra-articular infusion of a local anesthetic in a joint after surgery, several authors have observed a devastating complication termed postarthroscopic glenohumeral chondrolysis because the vast majority of these complications have occurred after shoulder surgery in young and previously healthy patients (athletes). Joint destruction is often rapid and severe, and there is no easy treatment available. Case series have identified that chondrolysis occurs mainly in patients receiving an intraarticular infusion of bupivacaine73 with increased toxicity when using larger concentrations. Addition of epinephrine may increase the toxic effect.74 Other local anesthetic drugs also possess chondrolytic properties but to a lesser degree. A long (i.e., ≥1 day) contact duration is needed for the effect to become severe. The effect may be linked to a proapoptotic effect and mitochondrial dysfunction induced by bupivacaine.75 This complication was not anticipated when the use of intra-articular infusion increased rapidly some years ago, suggesting that every new usage should be submitted to intense monitoring, even for drugs that are thought to be very well known.
Another devastating complication for which interest has recently increased is the occcurrence of ischemic cerebral complications after shoulder surgery (Chapter 6). This complication, although exceptional,76 should be known by all anesthesiologists who perform surgery in sitting (beach chair) position.77 At least two main mechanisms can lead to cerebral ischemia. The sitting position is associated with venous pooling in the lower limbs and reduces venous return to the heart, leading to bradycardia and hypotension. Moreover, because the arterial pressure cuff is placed on the upper limb, the blood pressure measured does not take into account the pressure gradient and the blood pressure is overestimated by nearly 20 mm Hg. When the pressure cuff is placed on the calf (often as a request from the surgeon), then overestimation may be ≥50 mm Hg (Figure 6-4). Hypotension is thus not detected and cerebral ischemia may occur. Adding an interscalene block may also increase the risk as this regional technique may further activate the Bezold-Jarisch reflex.78 Apart from limiting the indications of beach chair positioning, prevention relies mainly on integrating the blood pressure difference in the value seen on the screen to avoid prolonged hypotension. Murphy et al.79 have also shown recently that cerebral ischemia can be detected by continuous monitoring of cerebral near-infrared spectroscopy.
In recent years, a significant focus has been made on human factors that may contribute to errors (Chapter 3). Medication errors are among the most common causes and can occur at any time during the process, that is during delivery from the pharmacy, preparation of the solution, or during administration. The different strategies that are aimed at decreasing the rate (and severity) of medication errors are well described and not mutually exclusive.80,81
When RA is considered, general causes of errors can occur, but it has been shown that spinal administration of toxic drugs (not intended for spinal use) can have devastating consequences. Interestingly, in the UK, the National Patient Safety Agency has issued a Patient Safety Alert, with the aim of eliminating Luer connectors from equipment for lumbar puncture and subarachnoid injections by April 1, 2011, to reduce drug errors by using a mistake-proofing technique and avoiding Luer connections for all devices related to neuraxial anesthesia. The deadline has been recently updated to April 1, 2012, because technical solutions are not yet perfect.82 Whatever the need for update, the agency should be commended for this decisive action, which had not been taken by any other country before.
 SUMMARY
SUMMARY
The number of RA procedures has increased significantly since the turn of the 20th century, not only in relation with the increased number of surgical procedures but also as an increased proportion of anesthetic procedures. All types of regional anesthetic techniques are being more widely used, including the traditional (spinal and epidural) and more modern ones (PNB as single-shot or continuous techniques). In contrast with this increased use, which suggests an increased safety of these techniques, data from large-scale surveys do not yet show a decrease in the overall rate of complications. The unchanged incidence could be related to methodologic bias, but it is likely that overall safety has improved. Many strategies such as improved training, use of safer devices and drugs, technologic innovation, and the use of quality-improvement programs have indeed been implemented to control the risks of RA. This probably explains why severe complications are now very rare in healthy patients (e.g., obstetric patients). It is however possible that, in contrast to the general decrease in the rate of traditional complications, the trend might be reversed by the negative anatomical or physiologic effect of ageing which is also associated with an increased use of concomitant anticoagulation. Apart from these complications, our recent experience has seen the occurrence of new complications. Fortunately, because these new complications have been rapidly identified, it is to be expected that they will not have any significant effect on the overall slope. Complications related to anesthesia and especially those related to RA are often poorly accepted by patients because RA is viewed as a technique safer than general anesthesia and used in clinical conditions associated with comfort and pain control. If we wish that RA not undergo a new wave of blaming and litigation, major efforts to improve safety remain to be done. Improvement in techniques and training is mandatory but are not enough. Procedures should be better defined, and applied and system errors should be cured if one wants RA to become an ultra-safe technique.
References
- Clergue F, Auroy Y, Pequignot F, et al. French survey of anesthesia in 1996. Anesthesiology 1999;91:1509–1520.
- Auroy Y, Laxenaire MC, Clergue F, et al. Anesthetics in obstetrics. Ann Fr Anesth Reanim 1997;17:1342–1346.
- Burnstein R, Buckland R, Pickett JA. A survey of epidural analgesia for labour in the United Kingdom. Anaesthesia 1999;54: 634–640.
- Shibli KU, Russell IF. A survey of anaesthetic techniques used for caesarean section in the UK in 1997. Int J Obstet Anesth 2000;9:160–167.
- Memsoudis SG, Kuo C, Edwards AM, et al. Changes in anesthesia related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med 2011;36:327–331.
- Fox MAL, Webb RK, Singleton RJ, et al. Problems with regional anaesthesia: an analysis of 2000 incident reports. Anaesth Intensive Care 1993;21: 646–649.
- Kennedy F, Effron AS, Perry G. The grave spinal cord paralyses caused by spinal anesthesia. Surg Gynecol Obstet 1950;91: 385–398.
- Cope RW. The Wooley and Roe case. Anaesthesia 1954;9:249–270.
- Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology 1984;61:276–310.
- Capdevila X, Barthelet Y, Biboulet P, et al. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91:8–15.
- Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg 2005;100:1822–1833.
- Hadzic A, Williams BA, Karaca PE, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology 2005;102:1001–1007.
- Dripps RD, Vandam LD. Long-term follow-up of patients who received 10,098 spinal anesthetics: failure to discover major neurological sequelae. JAMA 1954;156:1486–1491.
- Phillips OC, Ebner H, Nelson AT, et al. Neurologic complications following spinal anesthesia with lidocaine: a prospective review of 10,440 cases. Anesthesiology 1969;30:284–289.
- Borgeat A, Ekatodramis G, Kalberer F, et al. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001;95:875–880.
- Auroy Y, Narchi P, Messiah A, et al. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology 1997;87:479–486.
- Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97:1274–1280.
- Gaba D. Safety first: ensuring quality care in the intensely productive environment—the HRO model. APSF Newsletter. Spring, 2003.
- Auroy Y, Benhamou D, Amaberti R. Risk assessment and control require analysis of both outcomes and process of care. Anesthesiology 2004;101:815–817.
- Reason J. Human error: models and management. BMJ 2000; 320:768–770.
- Aviation Safety Reporting System. Available at: http://asrs.arc.nasa.gov. Accessed July 14, 2004.
- Vincent C, Taylor-Adams S, Stanhope N. Framework for analysing risk and safety in clinical medicine. BMJ 1998;316:1154–1157.
- Wang LP, Hauerberg J, Schmidt JF. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology 1999;91:1928–1936.
- Vandam LD, Dripps RD. A long-term follow-up of 10,098 spinal anesthetics. II. Incidence and analysis of minor sensory neurological defects. Surgery 1955;38:463–469.
- Finegold H, Mandell G, Vallejo M, et al. Does spinal anesthesia cause hearing loss in the obstetric population? Anesth Analg 2002;95:198–203.
- Wong CA, Slavenas P. The incidence of transient radicular irritation after spinal anesthesia in obstetric patients. Reg Anesth Pain Med 1999;24:55–58.
- Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology 1990;72:828–833.
- Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979;51:285–287.
- Schneider MC, Hampl KF, Kaufmann M. Transient neurologic toxicity after subarachnoid anesthesia with hyperbaric 5% lidocaine. Anesth Analg 1994;79:610.
- Chazalon P, Tourtier JP, Villevielle T, et al. Ropivacaine-induced cardiac arrest after peripheral nerve block: successful resuscitation. Anesthesiology 2003;99:1449–1451.
- Huet O, Eyrolle LJ, Mazoit JX, et al. Cardiac arrest after injection of ropivacaine for posterior lumbar plexus blockade. Anesthesiology 2003;99:1451–1453.
- Rosenblatt MA, Abel M, Fischer GW, et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology 2006;105:217–218.
- Frankel HL, Crede WB, Topal JE, et al. Use of corporate six sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg 2005;201:349–358.
- Bollini CA, Urmey WF, Vascello L, et al. Relationship between evoked motor response and sensory paresthesia in interscalene brachial plexus block. Reg Anesth Pain Med 2003;28:384–388.
- Karaca P, Hadzic A, Yufa M, et al. Painful paresthesiae are infrequent during brachial plexus localization using low-current peripheral nerve stimulation. Reg Anesth Pain Med 2003;28:380–383.
- Hogan Q. Finding nerves is not simple. Reg Anesth Pain Med 2003;28:367–371.
- Holdcroft A, Gibberd FB, Hargrove RL, et al. Neurological complications associated with pregnancy. Br J Anaesth 1995;75:522–526.
- Wong CA. Neurologic deficits and labor analgesia. Reg Anesth Pain Med 2004;29:341–351.
- Wong CA, Scavone BM, Dugan S, et al. Incidence of postpartum lumbosacral spine and lower extremity nerve injuries. Obstet Gynecol 2003;101:279–288.
- Alexander JM, Sharma SK, McIntire DD, et al. Intensity of labor pain and cesarean delivery. Anesth Analg 2001;92:1524–1528.
- Nercessian OA, Macaulay W, Stinchfield FE. Peripheral neuropathies following total hip arthroplasty. J Arthroplasty 1994;9:645–651.
- DeHart MM, Riley Jr LH. Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg 1999;7:101–111.
- Ben-David B, Joshi R, Chelly JE. Sciatic nerve palsy after total hip arthroplasty in a patient receiving continuous lumbar plexus block. Anesth Analg 2003;97:1180–1182.
- Aminoff MJ. Electrophysiologic testing for the diagnosis of peripheral nerve injuries. Anesthesiology 2004;100:1298–1303.
- Kopacz DJ, Neal JM, Pollock JE. The regional anesthesia “learning curve.” What is the minimum number of epidural and spinal blocks to reach consistency? Reg Anesth 1996;21:182–190.
- Lascarrou JB, Thibaut F, Malinovsky JM. Cardiac arrest after axillary plexic anaesthesia with ropivacaine in a chronic kidney failure dialysis patient. Ann Fr Anesth Reanim 2008;27:495–498.
- Weinberg GL, Ripper R, Murphy P, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med 2006;31:296–303.
- Marhofer P, Eichenberger U, Stöckli S, et al. Ultrasonographic guided axillary plexus blocks with low volumes of local anaesthetics: a crossover volunteer study. Anaesthesia 2010;65:266–271.
- O’Donnell BD, Iohom G. An estimation of the minimum effective anesthetic volume of 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesthesiology 2009;111:25–29.
- Noble AB, Murray JG. A review of the complications of spinal anaesthesia with experience in canadian teaching hopsitals from 1959 to 1969. Can Anaesth Soc J 1971;18:5–17.
- Scott DB, Tunstall ME. Serious complications associated with epidural/spinal blockade in obstetrics: a two-year prospective study. Int J Obstet Anesth 1995;4:133–139.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–1999. Anesthesiology 2004;101:950–959.
- Hebl JR, Horlocker TT, Kopp SL, et al. Neuraxial blockade in patients with preexisting spinal stenosis, lumbar disk disease, or prior spine surgery: efficacy and neurologic complications. Anesth Analg 2010;111:1511–1519.
- de Sèze MP, Sztark F, Janvier G, et al. Severe and long-lasting complications of the nerve root and spinal cord after central neuraxial blockade. Anesth Analg 2007;104:975–979.
- Kane RE. Neurologic deficits following epidural or spinal anesthesia. Anesth Analg 1981;60:150–161.
- Wysowski DK, Talarico L, Bacsanyi J, et al. Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med 1998;338:1774–1775.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Executive summary: regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010;35:102–105.
- Osta WA, Akbary H, Fuleihan SF. Epidural analgesia in vascular surgery patients actively taking clopidogrel. Br J Anaesth 2010;104:429–432.
- van Veen JJ, Nokes TJ, Makris M. The risk of spinal haematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals. Br J Haematol 2010;148:15–25.
- Johnson ME, Uhl CB, Spittler KH, et al. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology 2004;101:1184–1194.
- Myers RR, Sekiguchi Y, Kikuchi S, et al. Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp Neurol 2003;184:606–614.
- Robards C, Hadzic A, Somasundaram L, et al. Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg 2009;109:673–677.
- Hadzic A, Dewaele S, Gandhi K, et al. Volume and dose of local anesthetic necessary to block the axillary brachial plexus using ultrasound guidance. Anesthesiology 2009;111:8–9.
- Brull R, McCartney CJ, Chan VW, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg 2007;104:965–974.
- Fredrickson MJ, Kilfoyle DH. Neurological complication analysis of 1000 ultrasound guided peripheral nerve blocks for elective orthopaedic surgery: a prospective study. Anaesthesia 2009;64:836–844.
- Barrington MJ, Watts SA, Gledhill SR, et al. Preliminary results of the Australasian Regional Anaesthesia Collaboration: a prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg Anesth Pain Med 2009;34:534–541.
- Liu SS, Zayas VM, Gordon MA, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth Analg 2009;109:265–271.
- Capdevila X, Pirat P, Bringuier S, et al. Continuous peripheral nerve blocks on hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients. Anesthesiology 2005;103:1035–1045.
- Bergman BD, Hebl JR, Kent J, et al. Neurologic complications of 405 consecutive continuous axillary catheters. Anesth Analg 2003;96:247–252.
- Morin AM, Kranke P, Wulf H, et al. The effect of stimulating versus nonstimulating catheter techniques for continuous regional anesthesia: a semiquantitative systematic review. Reg Anesth Pain Med 2010;35:194–199.
- Ilfeld BM, Mariano ER, Girard PJ, et al. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain 2010;150:477–484.
- Mariano ER, Loland VJ, Sandhu NS, et al. Comparative efficacy of ultrasound-guided and stimulating popliteal-sciatic perineural catheters for postoperative analgesia. Can J Anesth 2010;57:919–926.
- Anderson SL, Buchko JZ, Taillon MR, et al. Chondrolysis of the glenohumeral joint after infusion of bupivacaine through an intra-articular pain pump catheter: a report of 18 cases. Arthroscopy 2010;26:451–461.
- Dragoo JL, Korotkova T, Kanwar R, et al. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med 2008;36:1484–1488.
- Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am 2010;92:609–618.
- Friedman DJ, Parnes NZ, Zimmer Z, et al. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics 2009;32: 256.
- Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth 2005;17: 463–469.
- D’Alessio JG, Weller RS, Rosenblum M. Activation of the Bezold-Jarisch reflex in the sitting position for shoulder arthroscopy using interscalene block. Anesth Analg 1995;80: 1158–1162.
- Murphy GS, Szokol JW, Marymony JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg 2010;111: 496–505.
- Jensen LS, Merry AF, Webster CS, et al. Evidence based strategies for preventing drug errors during anaesthesia. Anaesthesia 2004;59: 493–504.
- National Patient Safety Agency. Seven steps to patient safety: the full reference guide. 2004;11. Available from http://www.nrls.npsa.nhs.uk/resources/collections/seven-steps-to-patient-safety/. Accessed January 23, 2011.
- Cook TM, Payne S, Skryabina E, et al. A simulation-based evaluation of two proposed alternatives to Luer devices for use in neuraxial anaesthesia. Anaesthesia 2010;65:1069–1079.
< div class='tao-gold-member'>



