156 Organ Toxicity of Cancer Chemotherapy
Substantial improvements in survival rates among cancer patients admitted to the intensive care unit (ICU) have been achieved over the last decade.1 Three factors have contributed to these advances: (1) better patient selection, following reports in the 1980s of dismal outcomes2,3 and ensuing recommendations that ICU admission be denied in many situations involving cancer patients4–6; (2) improved overall survival of cancer patients,7 owing to therapeutic innovations and measures to prevent infections and drug toxicity; and (3) recent advances in ICU management of acute respiratory failure1,8 and septic shock.9
Today, hospital mortality among cancer patients admitted to the ICU is approximately 50%, which is not higher than in other patient groups (e.g., chronic obstructive pulmonary disease, chronic heart failure, pancreatitis, extensive burns, etc.). In addition, characteristics such as neutropenia, autologous bone marrow transplantation, or progression of malignancy no longer predict mortality.10–12 New treatments such as granulocyte colony-stimulating factor (G-CSF) shorten the duration of bone marrow failure,13,14 thereby diminishing the risk of treatment-related infection, and medications that have limited toxicity can achieve remissions in patients initially considered as having relentlessly progressive disease.15–17 As a result of these major therapeutic advances, the number of cancer patients referred for ICU admission is increasing steadily, with infection and treatment-related toxicity being the most common reasons.18
 Pulmonary Toxicity
Pulmonary Toxicity
Bleomycin-Induced Lung Toxicity
Bleomycin is a glycopeptide antibiotic that has been used since the 1970s in a wide range of solid tumors (lung cancer, esophageal cancer, head and neck cancer, germ-cell tumors of the ovary and testis, Kaposi sarcoma) as well as Hodgkin’s disease and non-Hodgkin’s lymphoma. Bleomycin lung toxicity occurs in 2% to 46% of patients.19,20 Pneumonitis with diffuse infiltrates and fibrosis is the most typical manifestation, and it has a fatal outcome in 1% to 3% of cases.19,20 Mean time to onset is 4 months after bleomycin administration, but some cases can develop up to 10 years after completion of bleomycin treatment.21,22 Earlier lung toxicity is less common and is responsible for clinical and radiographic manifestations reminiscent of bronchiolitis obliterans or hypersensitivity pneumonitis.20
Available knowledge of the pathophysiology of bleomycin-induced lung toxicity stems mainly from animal models. Skin and lungs are main targets because of the lack of bleomycin-inactivating hydrolase in these organs.20 By increasing free radicals, bleomycin induces endothelial damage, nuclear factor-kappa B (NF-κB) stimulation, then proinflammatory and profibrosis cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β and IL-18, and transforming growth factor β (TGF-β).23,24 Subsequently, there is an influx of inflammatory cells and fibroblasts, and progression to lung fibrosis can occur.20
Established risk factors include the cumulative dose of bleomycin, although the toxic amount varies across patients, without any consensual threshold for toxicity but rather a linear relation between the bleomycin dose and the incidence of lung toxicity.20 Renal failure seems to be the most important risk factor for predicting lung toxicity, with a significant association between diffusing capacity of the lung for carbon monoxide (DLCO) and creatinine clearance.19 Other risk factors for bleomycin-induced lung fibrosis include age older than 70 years, tobacco use, concomitant radiation therapy to the chest, bolus administration, oxygen exposure (often during or after surgery), and concomitant use of G-CSF or other cancer chemotherapy agents exhibiting lung toxicity.19,20,25
A dry cough, dyspnea on exertion and then at rest, tachypnea, fever, and cyanosis are the earliest symptoms.20 Fine, crackling rales are heard over both lung bases, and later in the course, rhonchi or a friction rub may be found. Infiltrates in both lung bases are typically seen on the chest radiograph, and progression to diffuse interstitial fibrosis may occur.20 However, asymmetric or more focal images are seen. Computed tomography shows earlier changes consisting of subpleural linear and nodular opacities in the lung bases that may suggest lung metastases.26 Blood gas measurements show hypoxemia and hypocapnia, and lung function testing discloses a restrictive defect with decreases in vital capacity and in the DLCO.20 The diagnosis is often one of exclusion when lung metastases and infections are eliminated. No real pathognomonic histologic finding exists. The most characteristic lesions are interstitial inflammatory cell infiltration and fibrosis and squamous metaplasia of bronchiolar epithelium.19,20
To decrease the risk of bleomycin-induced lung toxicity, the total dose should be determined according to the patient’s risk-factor profile, the objective being to find the best compromise between minimizing toxicity and optimizing the anticancer effect. Suggested prophylactic agents include anti-TNF-α and anti-TGF-β antibodies, IL-1 receptor antagonists, and antioxidants such as dexrazoxane, pentoxifylline, amifostine, and diallyl sulfide.20,24 Curative treatment starts with discontinuation of all chemotherapy agents known to cause lung toxicity and with respiratory function support. Infection must be ruled out. Glucocorticoid therapy in a dose of 60 to 100 mg/d of methylprednisolone is usually given, although compelling proof of efficacy is lacking. This practice is warranted given the possibility of bronchiolitis obliterans–organizing pneumonia or hypersensitivity pneumonitis, both of which respond to glucocorticoid therapy.20 In survivors, the symptoms resolve completely, and respiratory function returns to normal.20
Methotrexate Pneumonitis
Methotrexate (MTX) is a cytotoxic agent belonging to the antimetabolite class. It blocks purine synthesis by inhibiting dihydrofolate reductase. Methotrexate is not only used in various solid tumors and hematologic malignancies but also in nonmalignant diseases such as rheumatoid arthritis and severe psoriasis. Acute or subacute pneumonitis simulating an infection, usually with interstitial involvement, occurs in 1% to 7% of patients receiving MTX.27 It may occur even at low doses.28 Toxicity mechanisms include up-regulation of the p38 MAPK pathway and inflammatory cytokines such as IL-1β and IL-8.29 The symptoms may develop gradually over several weeks or months and include dyspnea, dry cough, crackling rales, and less often, fever and headaches. Extrapulmonary manifestations may include erosive mucositis, rash, and hepatic cytolysis.27 Peripheral blood eosinophil counts are moderately and transiently elevated. Hypoxemia, a restrictive defect, and a decrease in DLCO are typically found. BAL fluid contains an abundance of cells, with a predominance of lymphocytes; the CD4/CD8 ratio varies, most notably with the time from MTX administration to respiratory symptom onset.30 Lung biopsy, use of which is declining, shows lymphocytic infiltration of the interstitial tissue and, rarely but distinctively, granulomas in areas of type II pneumocyte hyperplasia27 with a variable degree of lung fibrosis.
Other Anticancer Agents With Lung Toxicity
Gemcitabine is an antimetabolite used to treat solid tumors and hematologic malignancies. Although the bone marrow is the main target of gemcitabine toxicity (with at times profound myelosuppression), pulmonary toxicity occurs in 10% to 42% of patients.31 Age older than 65 years, previous lung disease, chest radiation, and concomitant treatment with another agent (especially bleomycin in Hodgkin’s disease) are risk factors. There are two clinical variants: (1) infusion-related reactions, usually mild, characterized by dyspnea or bronchospasm within hours of infusion and by favorable outcome with corticosteroids32; and (2) gemcitabine-induced pneumonitis characterized by pulmonary edema at the time of a capillary leak syndrome, diffuse alveolar damage, or alveolar hemorrhage.32 Dyspnea, fever, pulmonary infiltrates, and cough are the main symptoms.31 Although the mortality can reach 37%, great improvement can be obtained with corticosteroids.31,32
Cytarabine, an agent similar to gemcitabine, has a longer history of use in acute myelogenous leukemia in combination with anthracyclines. Respiratory failure of variable severity develops in 12% to 20% of patients within 2 weeks of cytarabine initiation.33 Noncardiogenic pulmonary edema and organized pneumonia are described, with favorable outcome under corticosteroids. However, differential diagnosis with infection, leukemic infiltrates, or heart failure is often difficult.33,34
Tyrosine kinase inhibitors are generally well tolerated. Imatinib, the main treatment for chronic myeloid leukemia (CML), is also effective in gastrointestinal stromal tumors. It frequently induces edema and weight gain. Dyspnea and cough, observed in up to 14% of treated patients, are often attributed to pulmonary edema and pleural effusion.32 However, interstitial pneumonitis, alveolar hemorrhage, or pulmonary fibrosis can also occur (0.2% and 1.3% of grade 3 and 4 in the chronic phase of CML). Inhibition of platelet-derived growth factor (PDGF) is one of the mechanisms. Corticosteroid therapy can be effective.35 This lung toxicity is also described with some epidermal growth factor (EGF) inhibitors used in solid neoplasm.32
Prognosis of acute promyelocytic leukemia (APL) dramatically improved until introduction of all-trans-retinoic acid (ATRA). However, differentiation syndrome (DS), also known as retinoic acid syndrome, can be a life-threatening complication of this molecule. In the most recent study, it occurs in 25% of patients, with a severe form in 50% of them.36 Unexplained fever, weight gain greater than 5 kg, edema, dyspnea, interstitial pulmonary infiltrates, pleuropericardial effusion, unexplained hypotension, and renal failure are the main diagnosis criteria. High white blood cell count greater than 5 × 109/L and abnormal creatinine level are risk factors. Dexamethasone is used to prevent and treat this syndrome. Mortality in severe forms is 11%.36
Finally, a few cases of interstitial pneumonitis have been reported with carmustine, cyclophosphamide, melphalan, procarbazine, chlorambucil, mitomycin, vinblastine, etoposide, hydroxyurea, taxanes, alkylating agents, platin derivatives, rapamycin analogs, and monoclonal antibodies to EGFR.32 An exhaustive list of drugs potentially responsible for lung toxicity and the corresponding clinical presentations can be found online at http://www.pneumotox.com.
 Cardiac Toxicity
Cardiac Toxicity
Anthracyclines are the main culprits of cardiac toxicity. Evidence of cardiac toxicity for other agents (taxanes, antimetabolites, alkylating agents, and spindle poisons) is limited to anecdotal case reports.37
Anthracycline-Induced Cardiac Toxicity
The anthracycline class—which includes doxorubicin, daunorubicin, epirubicin, idarubicin, and mitoxantrone—plays a major role in the treatment of many solid tumors (breast cancer, esophageal cancer, osteosarcomas) and hematologic malignancies (Hodgkin’s disease, non-Hodgkin’s lymphoma, acute leukemia). Anthracycline-induced myocardial toxicity can be life threatening or dose limiting, thereby affecting the prognosis of the disease by precluding optimal anticancer treatment.38
Anthracyclines induce cell death of dividing cells via inhibition of topoisomerase-2, intercalation to nucleus DNA, and production of free radicals.39 The myocardium is vulnerable to free radicals because antioxidant enzyme activity is weaker in myocytes than in other tissues (e.g., liver, kidney). The cumulative anthracycline dose is the main risk factor for cardiac toxicity (1%-5%, up to 550 mg/m2; 30% at 600 mg/m2; 50% at 1g/m2) with individual variation.40 Other risk factors include female gender, age at either end of the lifespan, black race, and Down’s syndrome.38 Opinions are divided regarding the roles of prior radiation therapy to the chest, lymphoma, preexisting heart disease, and a preexisting decrease in the left ventricular ejection fraction.38,41
Acute cardiotoxicity manifests as an acute myocarditis, namely a rapid deterioration in cardiac function during or within 1 week after the administration of anthracycline therapy, usually with reversal of the abnormalities after discontinuation of the drug.38 Ventricular or supraventricular rhythm disorders are common. Congestive heart failure with or without cardiogenic shock is the most common clinical presentation, although myocarditis or pericarditis may also occur.41 Adjustments in chemotherapy regimens have noticeably reduced the rate of acute cardiac toxicity, which now occurs in fewer than 1% of patients.41
Chronic cardiotoxicity is far more common. The subacute form is characterized by irreversible dilated cardiomyopathy within 1 year after anthracycline discontinuation.42–44 The delayed form develops insidiously after more than 1 year and runs a slowly progressive course.42–44 Long-term follow-up studies allow better evaluation of the prevalence of subclinical cardiotoxicity after anthracycline doses between 450 and 550 mg/m2. It can reach 27.6%, with a median follow-up of 8 years, and the risk of cardiac failure clearly increases over time.40,45
Patients develop systolic or diastolic dysfunction indistinguishable from heart failure due to other causes. Coronary artery disease is rare,45 so electrocardiographic (ECG) changes are nonspecific and include sinus tachycardia, flat T waves, QT prolongation, and low amplitudes. Ventricular tachycardia and supraventricular rhythm disorders have been reported in patients with acute cardiac toxicity.41 B-type natriuretic peptide (BNP) is under study but not validated.40 Echocardiography with tissue Doppler studies is the most widely used noninvasive and sensitive tool for monitoring and early detection of anthracycline cardiomyopathy.38,40,41 Diastolic dysfunction is often the earlier sign. However, myocardial scintigraphy with technetium-99m may be more informative than transthoracic echocardiography, notably in obese patients. Dobutamine stress echocardiography has also been suggested as a diagnostic tool.46 Finally, myocardial biopsy is an invasive diagnostic method whose sensitivity and specificity are controversial. Histologic analysis shows myofibril loss, dilation of the sarcoplasmic reticulum, and intracytoplasmic vacuoles in myocytes.41
Preventive Treatment
Epirubicin and idarubicin may be less likely to induce cardiotoxicity than the other anthracyclines. Continuous administration over several hours also seems to reduce the cardiotoxicity of anthracyclines. Available cardioprotective agents include dexrazoxane, an antioxidant that chelates iron.47 Finally, liposomal encapsulation of anthracyclines reduces their cardiotoxicity without altering their anticancer effects.48
 Hematologic Toxicity
Hematologic Toxicity
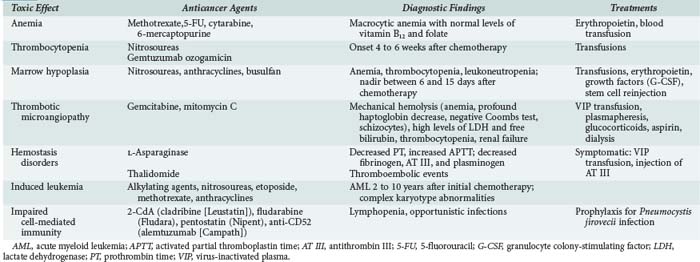
In addition to the myelosuppressive effects expected with all anticancer agents, alterations in hemostasis, impairments in cell-mediated immunity, and second leukemia or myelodysplasia can occur in patients with a history of chemotherapy for cancer (Table 156-1).
Myelosuppression
Myelosuppression is virtually unavoidable but usually reversible. The mechanism of action of the anticancer agent (i.e., the cell cycle phase affected by the drug) determines which cell lines are affected and governs the severity of marrow toxicity. For instance, nitrosoureas and mitomycin selectively destroy stem cells, causing severe and in some cases irreversible myelosuppression. In contrast, myelotoxicity is less marked with drugs that act more selectively on a specific cell-cycle phase, such as vincristine, bleomycin, and cisplatin. Table 156-2 recapitulates the severity of myelosuppression seen with various agents.
TABLE 156-2 Severity of Myelosuppression Seen with Various Chemotherapy Agents
| Mild | Moderate | Severe |
|---|---|---|
| Cisplatin | Antipurine | Anthracycline |
| Bleomycin | Podophyllin | Nitrogen mustard |
| Vinca alkaloids | Alkylating agents | Antifolates |
| Hydroxyurea | Antipyrimidines | |
| Mitomycin Procarbazine | Nitrosoureas (carmustine, lomustine) | |
| Busulfan | ||
| Dacarbazine |
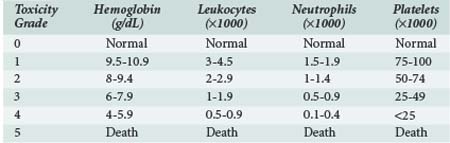
The severity of myelosuppression varies also with patient-dependent factors such as age, extent of bone marrow invasion by tumor, prior treatments (radiation therapy and/or chemotherapy associated with myelofibrosis), and nutritional status. The World Health Organization has suggested a scheme for classifying the severity of myelosuppression based on peripheral blood cell counts, as shown in Table 156-3.
< div class='tao-gold-member'>

Full access? Get Clinical Tree