212 Organ Donation After Cardiac Death
 Historical Perspective
Historical Perspective
The increasing gap between the number of organs available for transplantation and the number of patients listed for transplantation has become the rate-limiting step in reducing both wait times and wait-list deaths in patients with end-organ disease awaiting transplantation. Prior to the passage of the first U.S. brain death law in the state of Kansas in 1970,1 donation after cardiac death (DCD, or non-heartbeating donation) was the primary mode of organ donation in this country. Death in DCD donors was determined according to traditional cardiopulmonary criteria. Early organ procurement strategies were somewhat crude and variable, and consequently, warm ischemia time (time from donor circulatory arrest to cold perfusion) in the DCD donor was often prolonged and outcomes were poor.2 The impact of the type of graft on DCD outcomes was not apparent until experience with organs from donors declared brain dead (DBD) grew. Transplant centers in several states such as Nebraska, Ohio, North Carolina, and Illinois flourished after adoption of DBD in their respective states, and transplant volumes grew.3
The DBD phenomenon was a culmination of critical care physicians’ growing ability to maintain physiologic organ function in patients with little or no hope of neurologic recovery from severe insults to the central nervous system. A new debate was sparked over the precise definition and timing of death and the concept of futile care. This concept was introduced at a CIBA Foundation meeting in England in 1965 and subsequently endorsed with formal diagnostic criteria by Harvard Medical School in 1968.1,4 Acceptance of this medically, philosophically, and legally novel concept radically changed the face of transplantation. The revolutionary ability to certify death while perfusing the donor with oxygenated blood guaranteed procurement with minimal warm ischemia and graft damage and better recipient outcomes. As early experience with the DBD organs demonstrated superior outcomes, the use of DCD organs declined and was subsequently abandoned.5
As a result of the success seen with DBD organ donation, the number of U.S. transplants performed annually increased exponentially. Based on Organ Procurement and Transplantation Network (OPTN) data, in 1988, the first year for which reliable national data were available, 10,794 deceased-donor transplants were performed.6 Just 6 years later, annual volumes increased by nearly 50% to 15,210 total transplants. Most dramatically, the number of lung grafts from deceased donors increased from 33 to 708.6 Moreover, intestinal transplantation gained clinical success with the introduction of DBD donors (in addition to refined medical and surgical techniques). The first case was performed in 1990; by 1994, 96 patients with intestinal failure had received intestinal transplants.6 Concomitantly, advances in critical care resulted in reduced mortality in patients with end-stage organ disease, thereby resulting in increasing additions to and decreased attrition from the wait list, often referred to as the growing “gap” between supply and demand in transplantation. For example, despite a burgeoning number of transplant centers, rapid increase in transplants performed, and increased utilization of living donors, in 1995, only 33% of listed registrants waiting for kidney transplant (33,167) were transplanted (11,081).6 Unfortunately, the rate of transplantation fell to 10% of the list in the subsequent era of 1998 to 2002.7
Exacerbating the impact of this trend, numbers of young, previously healthy DBD donors stagnated due to several statutory changes in the areas of gun control, automobile safety (air bags, seat belts, lowering of legal blood alcohol limits), and cyclist helmet use, thereby reducing traumatic fatalities and consequently changing the face of DBD organ donors in the process.8 The demographics and mode of death of the typical DBD donor transitioned from a young, healthy person rendered brain dead as a result of a devastating head trauma toward an older person rendered brain dead from a neurovascular insult. The change in median donor age and mode of death ultimately eroded some of the benefit of utilization of the DBD donor and prompted a search for additional options.
As noted by DeVita, in 1993, the University of Pittsburgh Medical Center (UPMC) introduced the nation’s first institutional policy to permit and regulate DCD donation.9 The need for such a policy arose when several patients and their families asked to participate in organ donation after previously electing withdrawal of life-sustaining treatment. This was a request that fell outside the parameters of donation policies and guidelines then in effect. The UPMC policy became the first concrete model for the use of cardiopulmonary criteria to determine death for the purposes of organ procurement,10 and it highlighted a milestone in the evolution of the practice of transplantation in this country. Since, DCD utilization has been adopted by many organ procurement organizations (OPOs) and hospitals nationwide. By December 2006, OPTN bylaws required that all OPTN members have a DCD donor protocol in place.10 Moreover, the Joint Commission now requires that all accredited institutions develop and implement standardized DCD policies.11
 Current Status of DCD Donation
Current Status of DCD Donation
Volume
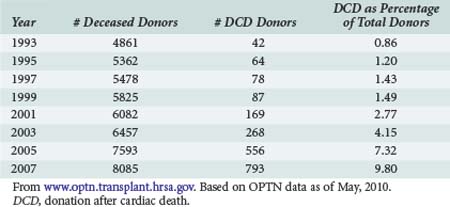
United Network for Organ Sharing (UNOS), the national nonprofit entity charged with disseminating both education and data pertaining to transplantation in the United States, has reported data on organs procured via DCD donation since 1994.6 Data are available via the OPTN website, www.optn.transplant.hrsa.gov/ and in OPTN annual reports. Table 212-1 demonstrates that the annual number of DCD organ donors increased steadily for the better part the mid 1990s to the early 21st century. The 188 DCD donor recoveries performed in 2002 represented 3% of total donors that year. In 2009, DCD recoveries represented 12% of all procurements, a fourfold increase from 2002.6
As a result of the OPTN and Joint Commission mandates mentioned earlier, the number of OPOs facilitating DCD recoveries in a given year has also risen overall, although not as sharply as the number of procurements performed: from 13 in 1993 to 33 in 2001. For the last year reported, 43 of the 59 OPOs facilitated at least one DCD procurement.7 The next logical question is whether the increased volume of DCD procurements has accordingly impacted transplant outcome metrics.
Outcomes
Though fraught with ethical controversy over the years, the real barrier to widespread acceptance of DCD graft utilization is based primarily upon the poor outcomes seen in the early DCD experience. Suboptimal organ function characterized by primary nonfunction (PNF), delayed graft function (DGF),12 and/or abbreviated graft survival have traditionally been a threat to success with DCD donors organs because of the warm ischemic insult associated with cardiopulmonary arrest. Although these observations were valid at the time, they were accumulated during the early experiences with transplantation and are thus inherently confounded by era bias.
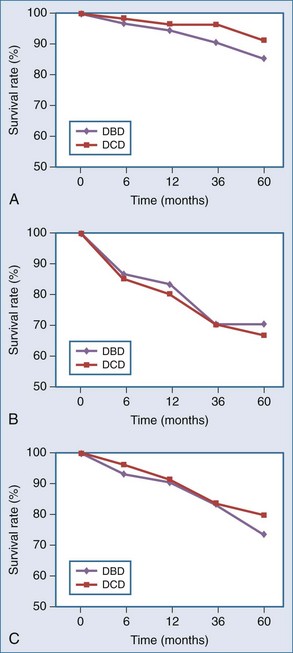
The primary lesson from the early DCD era was that the metabolically active renal cortex, biliary epithelium, pulmonary alveoli/central airways, and islets are sensitive to ischemia, with warm ischemic injury manifesting as acute tubular necrosis (ATN), ischemic-type biliary strictures (ITBS), bronchial dehiscence, and impaired beta cell function. These complications have been postulated to translate into and account for both poor initial graft function and long-term complications, seen particularly in the early era.12,13,14 Droupy and Abt, however, in separate studies, report that outcomes have improved; that intermediate and long-term patient/graft survival in recipients of controlled DCD kidney and liver grafts, respectively, are equivalent to or approach that of DBD.15,16 Per Droupy, DCD and DBD renal grafts followed for 10 years demonstrate equivalent survival despite a higher initial incidence of DGF for the DCD cohort. Salvaggio and colleagues, in an analysis of UNOS/OPTN data, concur17 (Figure 212-1, A).
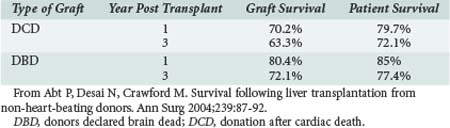
DCD liver outcomes have improved, though less dramatically. Some results of liver transplants using DCD donors were discouraging; both graft and patient survival rates were thought to be significantly lower when compared with DBD donors.18 Morbidity rates were higher as well.19,20 However, reviews isolating recent data—as, for example, data outlined by Abt—demonstrate that 1- and 3-year patient survival rates for liver DCD are now similar to those from DBD, although lower graft survival rates for DCD liver grafts persist16 (Table 212-2).
Much of the available outcome data for pancreatic DCD organs are derived from cases of simultaneous pancreas-kidney (SPK) transplants, demonstrating pancreatic graft and patient survival rates similar to those for DBD17 (see Figure 212-1, B). Utilization of pancreas-alone DCD grafts under the same protocols as SPK DCD grafts, while less frequent over the last decade, have been favorable17 (see Figure 212-1, C). Ongoing clinical experience will determine whether outcomes will reach those of SPK DCD grafts.
Oliveira et al. have, in the largest single-center series to date, demonstrated that lung grafts from DCD donors can also confer graft and patient survival rates equivalent to those from DBD donors.13 Of interest, these outcomes have been achieved in many settings in recipients who have been disproportionately more ill prior to transplant but deemed reasonable potential DCD recipients because of the long potential wait for DBD grafts. Consequently, clinicians now consider use of grafts that were previously routinely declined.21
 Identification and Categorization of the Potential DCD Donor
Identification and Categorization of the Potential DCD Donor
An important initial step in the process of DCD organ transplantation is recognizing the potential suitable donor. A significant consideration is the need to minimize organ ischemia in the presence of an unanticipated uncontrolled cardiac arrest; thus while organ procurement from DCD donors under uncontrolled conditions is technically feasible, it remains rare in contemporary practice.22,23 Similarly, graft quality is compromised in situations in which a patient’s wishes regarding organ donation are unknown. Organ suitability declines while attempts are made to locate family members to obtain consent. The once popular practice by some institutions to manage potential DCD donors brought to the emergency department by placement of vascular and/or intraperitoneal catheters in order to infuse cold organ preservation solution before consent for procurement became available24 has been largely abandoned. The practice stimulated contentious debate from opponents in both the medical and lay communities; unlike several European countries, no U.S. state at the time of the writing of this chapter has adopted presumed consent into law.
Remaining potential DCD donors are patients consented for donation with impending cardiopulmonary death, the timing of which is either unpredictable or predictable based upon patient/family-requested withdrawal of care, or unpredictable with premature arrest before withdrawal. Understandably, each type of DCD confers a varying risk of ischemic injury. A discussion of the management of DCD donors is facilitated by use of a classification scheme developed at a donor conference convened in 1994 by Maastricht, Netherlands, investigators.25 The Maastricht Categories define potential donors by the circumstances under which their cardiovascular death occurs. A distinction is made between those donors whose cardiopulmonary failure is uncontrolled or emergent (categories 1, 2, and 4) and those donors whose death by cardiopulmonary criteria occurs in a controlled, planned fashion by withdrawal of futile life-sustaining support (category 3). Maastricht Categories are outlined in Table 212-3.
TABLE 212-3 The Maastricht Classification for DCD Donors
| Category | Description | Condition |
|---|---|---|
| 1 | Cardiac arrest outside the hospital, no resuscitation attempted | Uncontrolled |
| 2 | Cardiac arrest followed by unsuccessful resuscitation, either inside or outside a hospital | Uncontrolled |
| 3 | Cardiac arrest after planned withdrawal of life-support technology | Controlled |
| 4 | Cardiac arrest in a brain-dead patient awaiting organ procurement | Uncontrolled |
From Koostra G, Daemen JHC, Oomen APA. Categories of non-heartbeating donors. Transplant Proc 1995;27:2893-4.
Of note, recent initiatives in the northeast United States involve training prehospital personnel in the rapid conversion of preconsented victims of unsuccessful resuscitation after cardiopulmonary arrest (category 2) to potential DCD donors.26 For the sake of uniformity, the remainder of this chapter will be devoted to discussion of category 3 donors.
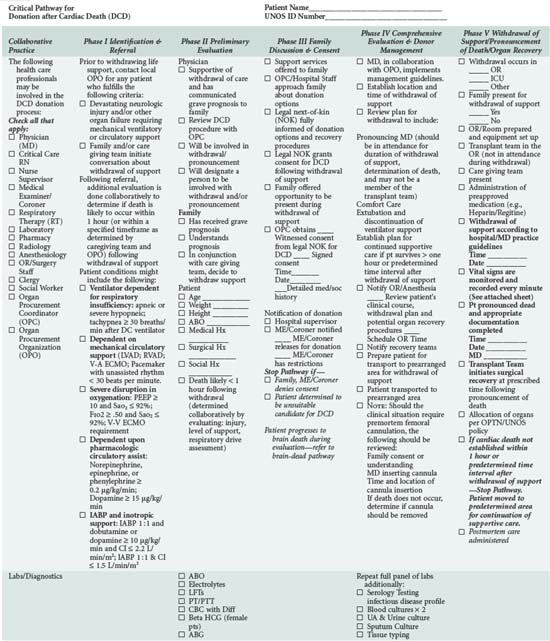
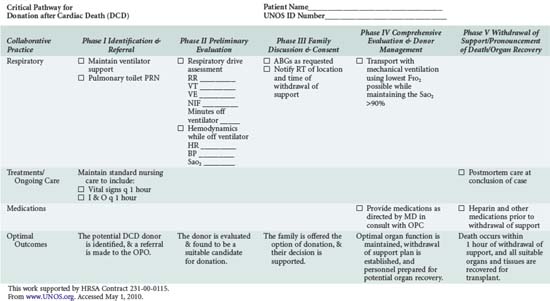
In addition to understanding the classification scheme and expected outcomes based upon absence or presence of controlled ischemia, the intensivist and OPO personnel must be familiar with the diagnoses and clinical circumstances qualifying a patient as a potential DCD donor. Again, candidates are patients in whom withdrawal of futile life-sustaining treatment is being planned. As shown in Table 212-4, the UNOS Critical Pathway for DCD,6 typical patients may have the following characteristics: absent or hyperactive respiratory drive, lack of adequate respiratory muscle strength, severe hypoxemia, or inadequate circulation in the absence of inotropic or vasopressor drugs. Such patients are usually supported by ventilators or mechanical circulatory assistance such as ventricular-assist devices (VAD) or intraaortic balloon pumps. They are often patients who have also suffered a severe neurologic insult. Conscious patients are usually suffering from degenerative neuromuscular diseases or end-stage cardiopulmonary disease and are often ventilator or VAD dependent. These patients or their families may decide to discontinue their support devices and request that their organs subsequently be donated.
< div class='tao-gold-member'>

Full access? Get Clinical Tree