Barbara M. Scavone
I. Physiologic Changes of Pregnancy
Pregnancy induces many physiologic changes, most of which are adaptations to support blood flow and oxygen delivery to the fetus.
A. Hematologic Changes
Blood volume increases by 40% to approximately 100 mL/kg; plasma volume increases by 30% to 50% and red blood cell volume by 20% to 30%, causing a physiologic anemia of pregnancy (Table 31-1). Blood viscosity is decreased, allowing easier flow to the fetus. Normal hemoglobin range during pregnancy is 10.5 to 14 g/dL and is lowest in the second trimester (Table 31-2).

The anemia of pregnancy is caused by a disproportionate increase in plasma volume relative to red cell volume.
Table 31-1 Summary of Physiologic Changes of Pregnancy at Term
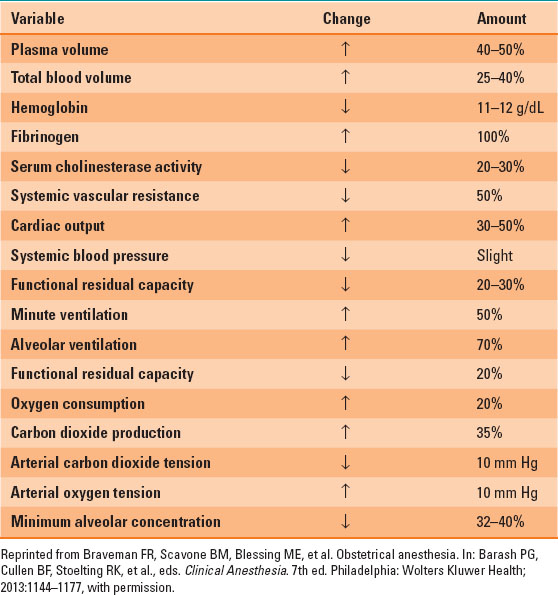
Table 31-2 Normal Lab Values in Pregnant Patients
Pregnancy is a prothrombotic state, and pregnant patients carry a greater risk of venous thromboembolism. Production of all clotting factors, except factors XI and XIII, increase. Fibrinogen levels significantly increase and are normally >400 mg/dL in the third trimester. A secondary fibrinolysis occurs later in pregnancy, and coagulation changes resemble a state of compensated disseminated intravascular coagulation (DIC). Platelet count may decrease during pregnancy due to dilution as well as increased consumption. Gestational thrombocytopenia is common, occurring in 8% of pregnancies, and is not associated with an increased risk of neuraxial hematoma.
Mild leukocytosis is normal during pregnancy. However, pregnancy is an immunosuppressed state, and pregnant patients do not tolerate the physiologic effects of systemic infection well, and mortality from sepsis is increased. In general, autoimmune diseases improve during pregnancy due to relative immunosuppression.
B. Cardiovascular Changes
Due to increased blood volume, stroke volume, and heart rate, cardiac output increases up to 45% by the end of the first trimester. During labor, cardiac output increases by another 50%, up to 80% over prelabor values immediately postpartum. Cardiovascular changes resolve several days postpartum. Systemic vascular resistance decreases, sometimes causing a mild decrease in blood pressure. Pregnant patients are less responsive to vasopressors and more sensitive to decreases in preload. After 20 weeks gestation, pregnant patients may experience supine hypotension syndrome when lying flat, because the gravid uterus can compress the IVC and decrease venous return. Therefore, LUD is the preferred position for pregnant patients over 20 weeks’ gestation.
 VIDEO 31-1
VIDEO 31-1
Left Uterine Displacement
C. Respiratory Changes
Oxygen consumption increases by 20% to 50% and minute ventilation by 50% at term. Minute ventilation increases mainly due to an increase in tidal volume and to a lesser extent respiratory rate. This physiologic hyperventilation decreases arterial partial pressure of carbon dioxide (PaCO2) levels to 28 to 32 mm Hg and may cause a slight increase in arterial partial pressure of oxygen (PaO2) levels. Compensatory metabolic acidosis occurs, bicarbonate levels are normally 18 to 22 mEq/L, and pH only slightly increases (7.45). Vital capacity and closing volume remain the same, but expiratory reserve volume and functional residual capacity decrease, making the pregnant patient quick to desaturate during periods of apnea, in particular in the supine position. A rightward shift in the hemoglobin–oxygen dissociation curve occurs, facilitating oxygen transfer to the fetus.

Left uterine displacement should be used for pregnant patients over 20 weeks’ gestation to prevent compression of the inferior vena cava by the gravid uterus.
D. Airway Changes
Mucosal edema and capillary engorgement occur as pregnancy progresses, and Mallampati class increases at term. The incidence of difficult mask ventilation and laryngoscopy also increases, with difficult or failed intubation occurring in one of 224 pregnant patients versus one of 2,500 in the general surgical population (1). A longer second stage of labor and preeclampsia are both associated with increased airway edema. Proper positioning and preoxygenation assume additional importance during induction of general anesthesia and intubation of pregnant versus nonpregnant patients (Fig. 31-1).

The normal arterial partial pressure of carbon dioxide during pregnancy is 28 to 32 mm Hg.
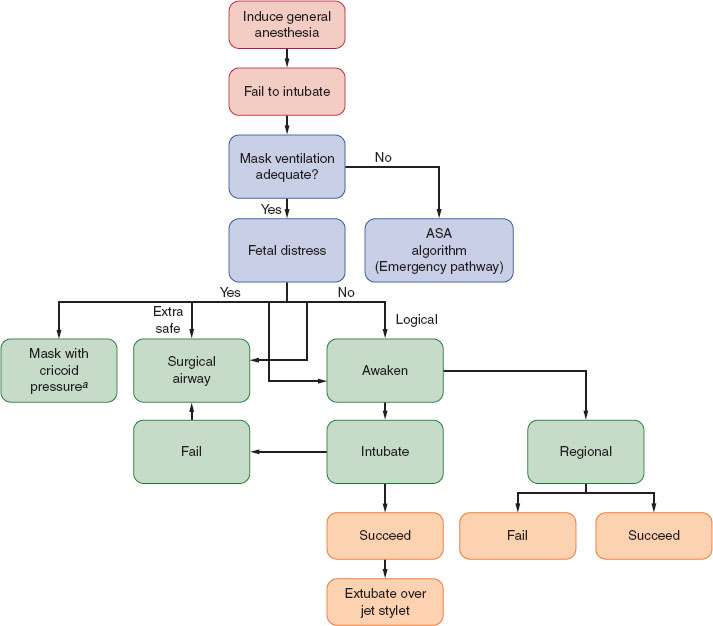
FIGURE 31-1 Management of the difficult airway in pregnancy with special reference to the presence or absence of fetal distress. When mask ventilation is not possible, the clinician is referred to the American Society of Anesthesiologists algorithm for the emergency airway management. aConventional face mask or laryngeal mask airway. (From Braveman FR, Scavone BM, Blessing ME, et al. Obstetrical anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:1144–1177, with permission.)
E. Gastrointestinal Changes
The gravid uterus causes mechanical gastroesophageal sphincter dysfunction. Progesterone decreases lower esophageal sphincter tone, predisposing pregnant patients to reflux of stomach contents into the oropharynx. In addition, the gravid uterus increases abdominal pressure and, thus, intragastric pressure, furthering reflux of stomach contents. Gastrin secretion by the placenta causes greater acidity of stomach contents. Finally, progesterone slows gastric emptying and gastrointestinal mobility. Pregnant women often have a gastric volume of over 25 mL with pH <2.5, both of which are associated with aspiration pneumonitis syndrome. Administration of a nonparticulate antacid is the only reliable way to change gastric content pH and possibly reduce the chance of aspiration pneumonitis syndrome should aspiration occur.

The incidence of difficult mask ventilation and laryngoscopy is greater in pregnant patients than in the general surgical population.
F. Neurologic and Musculoskeletal Changes
Minimum alveolar concentration (MAC) decreases by 40% during pregnancy, possibly due to elevated progesterone levels, and returns to baseline by 1 week postpartum. Pregnant women are also more sensitive to neuraxial local anesthetics and require lower doses than nonpregnant patients. Epidural space volume is decreased due to epidural vein engorgement, and cerebrospinal fluid (CSF) pH is decreased. Ligamentous relaxation occurs, and lumbar lordosis is accentuated, raising the intercristal line from L4-5 in nonpregnant patients to L3-4 in pregnant patients.
G. Endocrine Changes
Glucose Control
Pregnancy is a diabetogenic state, due to human placental lactogen’s anti-insulin effects. Routine screening for gestational diabetes is done via a carbohydrate load between 24 to 26 weeks. Women with gestational or pregestational diabetes are at greater risk for fetal macrosomia and pregnancy complications. Tight glucose control during labor is the standard of care for diabetic women, with goal blood sugar range between 80 to 110 mg/dL in order to avoid neonatal hypoglycemia.
Thyroid
Human chorionic gonadotropin is similar in structure to thyroid-stimulating hormone and causes thyroid hormone levels to increase during pregnancy. Estrogen stimulates production of thyroid-binding globulin, allowing more thyroid hormone to circulate.
H. Renal and Hepatic Changes
Blood flow to the kidneys and renal autoregulation remains unchanged during pregnancy as long as blood pressure remains stable. Increased glomerular filtration rate and decreased creatinine occur due to increases in cardiac output, and serum creatinine over 0.8 is abnormal. Proteinuria is common, as is asymptomatic bacteriuria. Ureteral tone decreases and asymptomatic bacteriuria can lead to pyelonephritis. Therefore, urine is screened for infection during routine prenatal visits.
Hepatic blood flow is not altered during pregnancy. Elevated alkaline phosphatase levels are seen due to secretion by the placenta. Liver transaminase levels are unchanged. Plasma osmolality is lower, leading to tissue edema. Pseudocholinesterase levels decrease, without much clinical significance. Clotting factor production increases. Cholestasis of pregnancy can occur due to the effects of estrogen, and causes pruritus and an increased risk of stillbirth.
II. Uteroplacental and Fetal Circulation
The uterus receives 15% of the cardiac output at term; normal uterine blood flow is 700 to 900 mL/min. Uterine blood flow is derived from the uterine arteries and additionally via collaterals from the ovarian and cervical arteries. Uterine (and therefore placental and fetal) blood flow is directly proportional to uterine perfusion pressure (defined as the difference between uterine arterial pressure vs. uterine venous pressure), and inversely proportional to uterine artery vascular tone. The uterine vasculature is maximally vasodilated during pregnancy. A lack of uterine autoregulation makes blood flow proportional to perfusion pressure. Uterine vasculature maintains responsiveness to vasoconstrictors. Increases in uterine smooth muscle tone constrict uterine vessels, decreasing flow.

Oxygen and carbon dioxide exchange occurs via simple diffusion; higher maternal PaO2 favors diffusion to the fetus, and fetal carbon dioxide is higher than maternal carbon dioxide, favoring diffusion back to the mother.
The fetus exchanges gases and nutrients with its mother via the placenta. Fetal placental villi containing fetal capillaries are bathed in maternal blood supplied by the spiral arteries, which are branches of the uterine arteries. A normal umbilical cord has three vessels: one vein containing oxygenated blood from the placenta and two arteries carrying deoxygenated blood and waste back to the placenta. A number of mechanisms of transport from mother to fetus and back exist, including simple diffusion (most common, due to fetal or maternal concentration gradient), transcellular transfer, and endocytosis and exocytosis. Oxygen and carbon dioxide exchange occurs via simple diffusion; higher maternal PaO2 favors diffusion to the fetus, and fetal CO2 is higher than maternal CO2, favoring diffusion back to the mother.
Fetal hemoglobin has greater O2 carrying capacity. The Bohr effect is more profound in the fetus because fetal hemoglobin encounters more hydrogen ions (H+) in the fetus, which is relatively more acidotic than in the mother, and is more likely to release O2 it is carrying to fetal tissues. Fetal circulation differs from adult circulation in that it bypasses the lungs (Fig. 31-2).
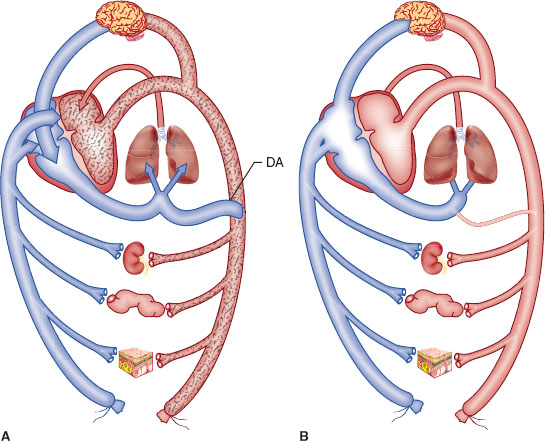
FIGURE 31-2 A: Schematic representation of the fetal circulation. Oxygenated blood leaves the placenta in the umbilical vein (vessel without stippling). Umbilical blood joins blood from the viscera (represented here by the kidney, gut, and skin) in the inferior vena cava. Approximately half of the inferior vena cava flow passes through the foramen ovale to the left atrium, where it mixes with a small amount of pulmonary venous blood. This relatively well-oxygenated blood (light stippling) supplies the heart and brain by way of the ascending aorta. The other half of the inferior vena cava stream mixes with superior vena cava blood and enters the right ventricle (blood in the right atrium and ventricle has little oxygen, which is denoted by heavy stippling). Because the pulmonary arterioles are constricted, most of the blood in the main pulmonary artery flows through the ductus arteriosus (DA) so the descending aorta’s blood has less oxygen (heavy stippling) than does blood in the ascending aorta (light stippling). B: Schematic representation of the circulation in the normal newborn. After expansion of the lungs and ligation of the umbilical cord, pulmonary blood flow and left atrial and systemic arterial pressures increase. When left atrial pressure exceeds right atrial pressure, the foramen ovale closes so all inferior and superior vena cava blood leaves the right atrium, enters the right ventricle, and is pumped through the pulmonary artery toward the lung. With the increase in systemic arterial pressure and decrease in pulmonary artery pressure, flow through the ductus arteriosus becomes left to right, and the ductus constricts and closes. The course of circulation is the same as in the adult. (From Hall SC, Suresh S. Neonatal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:1179, with permission.)
The majority of maternally administered drugs will be delivered to the fetus via the placenta. The fetal/maternal ratio describes the concentration of drug in the fetal umbilical vein versus maternal serum concentration. Nonionized, nonprotein bound, lipid-soluble drugs with molecular weights below 600 Da easily cross the placenta. Large, ionized, hydrophilic drugs are less likely to transfer. Most anesthetic drugs cross the placenta, with the exception of paralyzing agents and glycopyrrolate. Heparin and insulin also do not cross the placenta. Transient fetal or neonatal depression can be seen after administration of induction agents, anesthetic gases, opioids, and benzodiazepines. The long-term effects of general anesthetic agents on neonatal outcome are unknown. Theoretically, nitrous oxide can interfere with DNA synthesis via oxidation of vitamin B12. However, in animal studies only prolonged (>24 hours) exposure to high-concentration nitrous oxide produces fetal loss.

Most anesthetic drugs cross the placenta, with the exception of paralyzing agents and glycopyrrolate. Heparin and insulin also do not cross the placenta.
III. Pain Pathways in Labor, Anatomy of the Spine, and Neuraxial Analgesia and Anesthesia
Pain is transmitted via different means in different stages of labor. Pain during the first stage of labor, which commences with the beginning of regular contractions and cervical dilation and ends at complete cervical dilation, is transmitted via visceral afferent fibers entering the spinal cord from T10-L1. During the second stage, which begins with complete cervical dilation and ends with delivery of the fetus, additional pain is caused by stretching of vaginal and perineal tissues and is transmitted via sacral somatic fibers. The third stage of labor begins after delivery of the fetus and ends with delivery of the placenta, and pain during this stage is also transmitted via sacral somatic fibers.

Pain during the first stage of labor, which commences with the beginning of regular contractions and cervical dilation and ends at complete cervical dilation, is transmitted via visceral afferent fibers entering the spinal cord from T10-L1.
The spine has 33 levels: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal. Skin dermatomal levels correspond to the vertebral level at which their nerve roots enter (Fig. 31-3). The spinal cord is protected by the bony spine, ligaments, and layers of connective tissue and is bathed in CSF. The spinal cord is covered by three membranes, called the spinal meninges. The outermost layer is the dura mater, deep to the dura is the arachnoid, under the arachnoid layer lies CSF, and the pia mater is adherent to the spinal cord. The spinal cord extends from its inception off the brainstem through the foramen magnum and continues to L1 in most adults. About 10% of adults have spinal cords that terminate lower, at L3. It is therefore prudent to perform neuraxial procedures below this level.
 VIDEO 31-2
VIDEO 31-2
Labor Pain Pathways
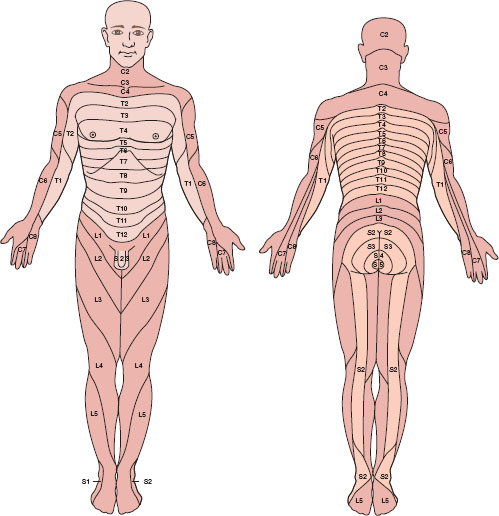
FIGURE 31-3 Human sensory dermatomes. (From Bernards CM, Hostetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:905–933, with permission.)
The spinal cord terminates as the cauda equina and the dural sac extends to S2. The epidural space spans from the foramen magnum to the sacral hiatus. It is a potential space and contains nerve roots, fat, valveless veins, lymphatics, and spinal arteries (Figs. 31-4 and 31-5).
 VIDEO 31-3
VIDEO 31-3
Subarachnoid Block Baricity
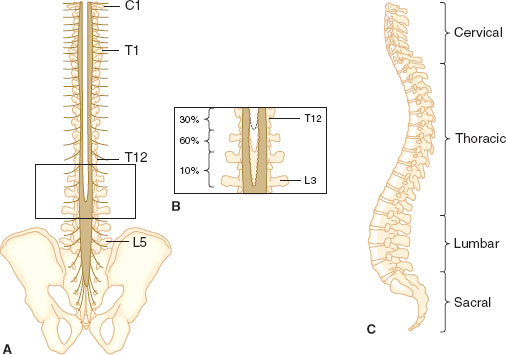
FIGURE 31-4 Posterior (A) and lateral (C) views of the human spinal column. Note the inset (B), which depicts the variability in vertebral level at which the spinal cord terminates. (From Bernards CM, Hostetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:905–933, with permission.)
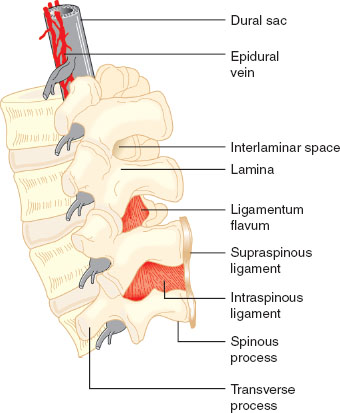
FIGURE 31-5 Detail of the lumbar spinal column and epidural space. Note that the epidural veins are largely restricted to the anterior and lateral epidural space. (From Bernards CM, Hostetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:905–933, with permission.)
Neuraxially administered local anesthetics cause blockade of sympathetic, sensory, and motor input and, depending on the dose, can provide analgesia or complete anesthesia. Small, myelinated, rapidly firing, active nerve fibers are more sensitive to local anesthetic blockade than larger, unmyelinated fibers. Degree of blockade from highest to lowest after administration of neuraxial local anesthetic is as follows: temperature sensation, vasomotor tone, sensory, and finally motor. Spinal anesthesia occurs via direct action of local anesthetic on the spinal cord, and block level depends on several factors, of which baricity and dose are the most significant.
Epidural anesthesia occurs via local anesthetic action on nerve roots and, to a lesser extent, has a direct effect on the spinal cord, via diffusion of local anesthetic into the intrathecal space. Usually 1 to 2 mL of epidural local anesthetic is required per lumbar dermatomal level requiring blockade.

The goal of avoiding excessive motor blockade, while still providing adequate analgesia, is commonly accomplished by administering low-concentration (0.0625% to 0.125% bupivacaine), high-volume, patient-controlled epidural anesthesia, with small amounts of opioid in the solutions.
Neuraxial labor analgesia provides excellent pain relief without effects on fetal or labor outcomes, with the exception of slightly increasing the length of the first and second stages of labor and the risk of instrumented vaginal delivery (2). Avoiding excessive motor blockade, while still providing adequate analgesia, is ideal in labor. This goal is commonly accomplished by administering low concentration (0.0625% to 0.125% bupivacaine) high-volume, patient-controlled epidural anesthesia (PCEA), with small amounts of opioid in the solutions.
After history, physical examination, and determination that the patient is a candidate for neuraxial anesthesia (Table 31-3), preparation for placement of a neuraxial block begins. A functioning intravenous line, resuscitation equipment, and blood pressure and heart rate monitoring are required, as are sterile precautions including hat, mask, hand hygiene, and sterile gloves. The patient is placed in either the sitting or lateral decubitus position, and the desired lumbar level is identified by palpation of the iliac crests and spinous processes. Most neuraxial catheters for labor are placed between L2-3 to L5-S1. After sterilizing the back with an antiseptic and placing a sterile drape, the anesthesiologist places a skin wheal of local anesthesia at the intended needle placement site. Either a midline or paramedian approach to the epidural space is possible. Midline is more common and easier to perform for beginners. Layers traversed by the epidural needle during placement of epidural block include, from superficial to deep, skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, and ligamentum flavum. The epidural space is a potential space lying deep to the ligamentum flavum. The hollow, large-bore epidural needle with stylet is placed into the superficial ligaments, the stylet is removed, and an air- or saline-filled syringe is attached. The syringe will give tactile resistance when pushed until the ligamentum flavum is traversed, and the epidural space is entered, at which time tactile resistance disappears. Both the thickness of the ligamentum flavum and depth of the epidural space are usually 3 to 5 mm. Once the space is entered, saline may be injected to confirm loss of resistance, and the depth at which the epidural space was entered is noted (most epidural needles have centimeter markings on them). A soft catheter is then threaded into the space 3 to 5 cm. A test dose of lidocaine mixed with 15 μg of epinephrine is commonly administered via the catheter to ensure that it is not intravascular or intrathecal. Criteria for a positive intravascular test dose include an increase in heart rate by 20 beats per minute or an increase in systolic blood pressure by 15 mm Hg within 45 seconds of administration. The catheter must be removed and replaced at the same or a different interspace if a positive intravascular test occurs. A profound sensory and motor block within 5 minutes after administration of test dose confirms a positive intrathecal. If an intrathecal catheter is accidentally placed, it may be used for labor analgesia with appropriate dosing or it may be removed and replaced at a different level (Fig. 31-6).
Table 31-3 Contraindications to Neuraxial Block

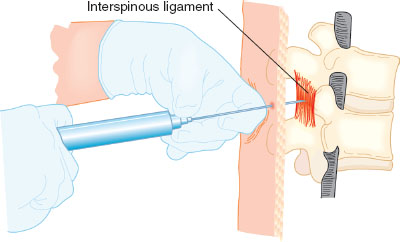
FIGURE 31-6 Proper hand position when using the loss-of-resistance technique to locate the epidural space. After embedding the needle tip in the ligamentum flavum, a syringe with 2 to 3 mL of saline and an air bubble is attached. The left hand rests securely on the back and the fingers of the left hand grasp the needle firmly. The left hand advances the needle slowly and under control by rotating at the wrist. The fingers of the right hand maintain constant pressure on the syringe plunger but do not aid in advancing the needle. If the needle tip is properly engaged in the ligamentum flavum, it should be possible to compress the air bubble without injecting the saline. As the needle tip enters the epidural space, there will be a sudden loss of resistance and the saline will be suddenly injected. (From Bernards CM, Hostetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health; 2013:905–933, with permission.)
Epidural activation begins with an initial bolus of dilute local anesthetic mixed with lipid soluble opioid, such as 0.125% bupivacaine mixed with fentanyl 50 to 100 μg, given in 5-mL increments, for a total of 10 to 20 mL. Time to analgesia is typically 10 to 20 minutes.
Alternatively, combined spinal epidural (CSE) has gained popularity because time to initial analgesia is shorter (3

Full access? Get Clinical Tree







