Chapter 43 Obesity, Sleep Apnea, the Airway, and Anesthesia
II. Definitions of Obesity and Obstructive Sleep Apnea
III. Pathophysiology of Obstructive Sleep Apnea
IV. Diagnosis of Obstructive Sleep Apnea
V. Obesity, Obstructive Sleep Apnea, and the Airway
VI. Effects of Anesthesia and Surgery on Postoperative Sleep
VII. Perioperative Risks of Obesity and Obstructive Sleep Apnea
VIII. Intraoperative Considerations
IX. Postoperative Considerations
I Introduction
Up to 80% of people with obstructive sleep apnea (OSA) are obese, and with obesity at epidemic proportions worldwide,1 OSA remains a major contributing factor to airway management difficulties. Numerous studies have reported major respiratory complications, including brain damage and death, in surgical patients with OSA.2,3 These disastrous outcomes result from failure to secure the airway during the induction of anesthesia, airway obstruction immediately after tracheal extubation, and respiratory arrest after the administration of opioids or sedation in the postoperative period.
The prevalence of OSA is about 20% in the U.S. population. It occurs as a result of partial or complete airway obstruction during sleep,4 and it is associated with episodic hypoxemia and hypercarbia.5–7 However, with the U.S. population aging and becoming obese, the prevalence of OSA is expected to increase significantly. Among the surgical population, patients with morbid obesity and OSA tend to be overrepresented due to the higher rates of obesity and OSA-related complications requiring surgical therapy.3,8 More than 75% of patients with OSA are undiagnosed or untreated.
II Definitions of Obesity and Obstructive Sleep Apnea
Obesity is a degree of excess weight associated with adverse health consequences.9 It is defined as a body mass index (BMI, or weight in kg divided by height in m2) greater than 29, and overweight is defined as a BMI of 25 to 29.9.9 A BMI of 40 or greater is classified as morbid obesity, and a BMI of 50 or greater designates super-obesity. Morbid obesity is associated with an increased risk of comorbidities,10 which may influence perioperative morbidity and mortality.11
OSA is a disorder characterized by repetitive upper airway collapse during sleep. Airflow ceases for more than 10 seconds, five or more times per hour, despite continuing ventilatory effort. It usually is associated with a decrease in arterial oxygen saturation (SaO2) of more than 4%.12 Obstructive sleep hypopnea is a greater than 50% decrease in airflow for more than 10 seconds occurring 15 times or more per hour of sleep. It is usually associated with a decrease of 4% or more in SaO2.
III Pathophysiology of Obstructive Sleep Apnea
A Pharyngeal Muscle Activity and Airway Patency
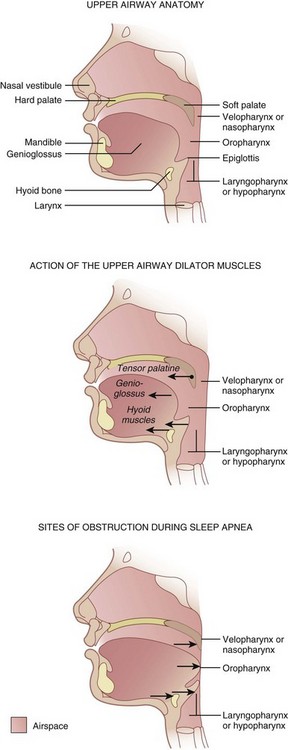
Three pharyngeal segments—nasopharynx (i.e., retropalatal pharynx), oropharynx (i.e., retroglossal pharynx), and laryngopharynx (i.e., retroepiglottic pharynx)—form the upper airway, which is a long, soft-walled tube that lacks bony support on the anterior and lateral walls, which makes it collapsible (Fig. 43-1).6 The transmural pressures across the pharyngeal walls (i.e., difference between extraluminal and intraluminal pressure) determine the patency of the upper airway. Activation of pharyngeal dilator muscles—the tensor veli palatini, the genioglossus, and the muscles of the hyoid bones (geniohyoid, sternohyoid, and thyrohyoid)—during inspiration counteracts the narrowing effects of reduced intraluminal pressure associated with inspiration. In addition to this inspiration-associated activation, tonic activity of these muscles during wakefulness helps to stabilize the pharyngeal walls.
B Sleep Pattern, Airway Obstruction, and Arousal
Normal sleep consists of four to six cycles of non–rapid-eye-movement (NREM) sleep followed by rapid-eye-movement (REM) sleep. The four stages of NREM sleep and one stage of REM sleep represent progressive slowing of the electroencephalographic waves. Rhythmic activity of the upper airway muscles decreases during deeper stages of sleep, which is accompanied by significant increase in upper airway resistance and consequent upper airway collapse.13
Contraction of the diaphragm during inspiration creates a subatmospheric pressure within the airway that may lead to narrowing of the collapsible segments of the pharynx.14 As pharyngeal pressure becomes more negative, pharyngeal collapse progressively increases. The lateral pharyngeal walls, a major site of pharyngeal adipose tissue deposition, are the most compliant and therefore the most common site of pharyngeal collapse.15 In obese patients, deposition of fat around the pharyngeal walls narrows the upper airway and increases the extraluminal pressure and collapse.16,17 For a given degree of loss of pharyngeal muscle tone and pharyngeal muscle collapse, a greater degree of pharyngeal obstruction is observed in patients with a posteriorly set tongue (caused by micrognathia and retrognathia or a receding mandible), large tongue, large tonsils, and nasal obstruction. Other factors that contribute to upper airway narrowing and subsequent collapse during sleep include: large neck circumference, anatomic or craniofacial abnormalities affecting the airway, and age.18,19
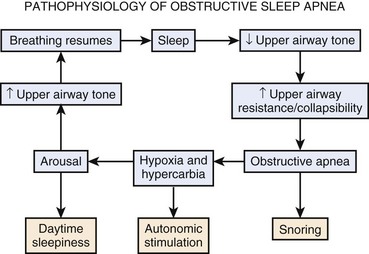
Airway collapse leads to obstructive apnea and consequently causes a decrease in arterial oxygen tension (PaO2) and increase in arterial carbon dioxide tension (PaCO2), which increases neural traffic in the reticular activating system, progressively increasing ventilatory efforts20,21 and causing arousal from sleep. Arousal, expressed as extremity twitching, gasping or snorting, vocalization, and increased electroencephalographic activity, reactivates the pharyngeal muscles and opens the upper airway. As the upper airway opens, ventilation resumes, which corrects hypoxia and hypercarbia.22 Hyperventilation after arousal reverses the blood gas disturbance to correspondingly decrease the central drive. The cycle repeats itself when the patient again falls asleep (Fig. 43-2).
Frequent arousals result in sleep disruption and excessive daytime somnolence, and oxygen desaturation, sympathetic hyperactivity, and a systemic inflammatory response may contribute to cardiovascular comorbidities such as systemic hypertension, cardiac arrhythmias, myocardial ischemia, pulmonary hypertension, and heart failure.23
IV Diagnosis of Obstructive Sleep Apnea
Because OSA is undiagnosed in an estimated 60% to 70% of patients and failure to recognize OSA preoperatively is one of the major causes of perioperative complications,5–7 all patients must be screened for OSA. Obtaining a thorough history and physical examination helps to determine a presumptive diagnosis of OSA, and polysomnography can confirm the diagnosis and severity of OSA and can determine the need for and level of continuous positive airway pressure (CPAP).
A Clinical Diagnosis
A systematic review and meta-analysis of clinical screening tests for OSA reported that the STOP-BANG screening tool was easy to use and a good predictor of severe OSA (i.e., apnea-hypopnea index [AHI] >30) (Box 43-1).24,25 The STOP-BANG questionnaire has a sensitivity of 93% and specificity of 43% at an AHI greater than 15 and a sensitivity of 100% and specificity of 37% at an AHI greater than 30.25 Other questionnaires, including the Berlin questionnaire and the American Society of Anesthesiologists (ASA) checklist, are also in clinical use and have a similar predictive accuracy for OSA.26–28
Box 43-1
STOP-BANG Scoring System
S = Snoring. Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?
T = Tiredness. Do you often feel tired, fatigued, or sleepy during daytime?
O = Observed apnea. Has anyone observed you stop breathing during your sleep?
P = Pressure. Do you have or are you being treated for high blood pressure?
From Chung F, Yegneswaran B, Liao P, et al: STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 108:812–821, 2008.
When the cricomental space, defined as the perpendicular distance from a line between the cricoid cartilage and the inner mentum to the skin of the neck, is more than 1.5 cm, the diagnosis of OSA can be excluded with a negative predictive value of 100%.29 A decision rule developed to diagnose OSA using three predictors (i.e., cricomental space ≤1.5 cm, pharyngeal grade greater than II, and presence of an overbite) has a positive predictive value of 95% and may provide an alternative to polysomnography.29
B Polysomnography
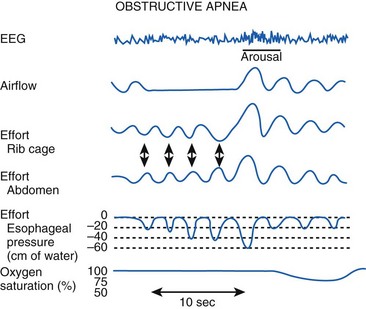
Polysomnography remains the gold standard in the diagnosis of OSA. Polysomnography consists of monitoring the electroencephalogram (EEG), electrooculogram (EOG), and submental electromyogram (EMG) for staging sleep. Oral and nasal airflow, respiratory efforts (i.e., inductance or impedance pneumography to monitor thoracoabdominal motion or the diaphragmatic EMG), oximetry, and capnography are also monitored. Body position, sound, arterial blood pressure, and the electrocardiogram are monitored (Fig. 43-3).30
The results of a sleep study are reported as events and indices. Events include apnea (no airflow ≥10 seconds), hypopnea (tidal volume [VT] ≤50% of the control awake value ≥10 seconds), desaturation (>4% decrease in SaO2), and arousal, which may be detected clinically (i.e., vocalization, turning, or extremity movement) or by an electroencephalographic burst.12 Indices are measured as events per hour, which include the AHI (i.e., number of times a patient was apneic or hypopneic per hour), oxygen desaturation index (i.e., number of times a patient had a more than 4% decrease in SaO2 per hour), and arousal index (i.e., number of times a patient was aroused per hour). If the patient has OSA, the entire sleep study is repeated with CPAP titration to determine the level of CPAP that causes a significant decrease in the AHI.
Because polysomnography may not always be available, other screening devices with single or multiple channels have been explored and may represent alternative methods to diagnose OSA. One study suggested that an O2 saturation value of more than 94% on room air in the absence of other causes should lend consideration to the diagnosis of long-standing OSA.31 The American Academy of Sleep Medicine recommended that the portable monitoring used as an alternative to a polysomnogram must record airflow, respiratory effort, and blood oxygenation. The device also must allow display of raw data with a capability for manual scoring or editing of automated scoring.32
It is unclear whether a routine preoperative sleep study (i.e., polysomnogram or home sleep study) could improve perioperative outcomes, because the optimal duration of preoperative CPAP therapy before proceeding with elective surgical procedures is unknown, and compliance with CPAP varies. For those suspected of having OSA based on clinical criteria, anesthesiologists may elect to proceed with a presumptive diagnosis of OSA, unless the patients have significant comorbidities.5,7
V Obesity, Obstructive Sleep Apnea, and the Airway
Obesity (determined by the BMI) is considered a predictor of difficult mask ventilation (DMV) and difficult intubation (DI).33 Morbidly obese patients have deposits of excess adipose tissue in the neck, breast, thoracic wall, and abdomen that may impede patency of and access to the upper airway. Magnetic resonance imaging studies of obese patients found greater amounts of fat in areas surrounding the collapsible segments of the pharynx in those with OSA,34,35 which may explain the difficulty in airway management in obese patients with OSA but not in all obese patients. The distribution pattern of body fat may be a more relevant factor contributing to difficult airway management than the BMI itself.34 Clinical studies have found that BMI alone is not a good predictor of a difficult airway.36–40
Patients with severe OSA (AHI ≥40) have been shown to be at a significantly higher risk for DMV and DI, leading to speculation that they may have different anatomic characteristics compared with patients who have less severe OSA.41 Obese patients with OSA have larger neck circumferences than equally obese patients (i.e., similar BMI) without OSA.16,42 This neck “mass loading” (up to 28% increase in neck soft tissue) may be responsible for a more collapsible airway, leading to DMV and DI.16 Men have a higher percentage of soft tissue and fat in the neck compared with women,43,44 which may explain greater airway difficulties in male OSA patients compared with female OSA patients. A logistic regression model identified neck circumference at the level of the thyroid cartilage as the single most predictor of problematic intubation.45 Probability of a DI increases significantly with a neck circumference of 40 cm or more.45,46 Neck circumference corrected for height (i.e., neck circumference/height) is sensitive and specific for detecting OSA compared with neck circumference alone.47 Racial differences in craniofacial anatomy may contribute to the severity of OSA and to a DI.48,49 Other factors that may contribute to difficult airway management include diabetes mellitus and abnormal facial morphology.50,51
Ultrasonography has been used to quantify neck soft tissue at the level of the vocal cords and suprasternal notch to determine potential predictors of difficult laryngoscopy in morbidly obese patients.52 The amount of pretracheal soft tissue was found to be a strong measure distinguishing an easy laryngoscopy from a difficult one.52 Lateral head and neck radiography can easily identify caudal soft tissue displacement, which shifts the hyoid bone caudally to increase the distance between the mandible and the hyoid bone. When this distance is more than 20 mm, the presence of OSA and a possible difficult airway should be suspected.53 Radiographic evaluation has affirmed strong relationships among DI and higher Mallampati scores, OSA, greater mandibular depth, and smaller mandibular and cervical angles.54
VI Effects of Anesthesia and Surgery on Postoperative Sleep
Other factors that influence sleep patterns and can exacerbate sleep disorders include the stress response to surgical insult and postoperative anxiety, pain, and opioids.55 These factors reduce REM sleep in the immediate postoperative period, which is followed by a rebound REM sleep that can last for several days after surgery.56 The rebound REM sleep makes patients with OSA even more vulnerable to airway obstruction. Postoperative sleep disturbances appear to be related to the location and invasiveness of the surgical procedure.57 For example, fewer sleep disturbances occur after mild or moderately invasive surgery than after major surgical procedures.
VII Perioperative Risks of Obesity and Obstructive Sleep Apnea
Factors that determine the perioperative risks in obese and OSA patients include the degree of obesity (i.e., BMI) and the severity of OSA, invasiveness of anesthesia and surgery, and postoperative opioid requirements.27 The ASA practice guidelines propose a scoring system that may be used to estimate whether an OSA patient is at increased risk for perioperative complications27 and to determine perioperative management (Box 43-2).