Nutritional Intervention
Jodie A. Bryk
Juan B. Ochoa
I. Introduction
Nutrition intervention (NI) is integral to the care of the surgical patient and is comparable to any other form of medical intervention. The biologic beneficial effects of NI are translated into better outcomes including decreased infection, improved healing of surgical and traumatic wounds, while decreasing cost and minimizing waste of health care resources. Similar to any therapy, NI has contraindications, risks and side effects, which need to be carefully prevented or promptly identified and managed. Knowledge of NI is progressing at a rapid pace and requires the surgeon maintain continuing education in this area.
Progress in both basic sciences and process-improvement applications are significant and have clarified the therapeutic role of NI, the metabolic effects of specific nutrients as well as the timing, route, and volume that NI should be delivered. Nutrition intervention in trauma and major abdominal surgery provides unique challenges.
The traditional goal of NI has focused on minimizing nitrogen loss and achieving a positive nitrogen balance (Fig. 8-1). In the past few years, investigators have moved beyond these goals realizing that:
Positive nitrogen balance can only be achieved through a better understanding and regulation of the immune response to injury or critical illness. Catabolism seen in trauma, sepsis, and stress cannot be curtailed by exceeding caloric requirements.
Higher than normal amounts of protein are necessary after injury. This may require modular additions of protein to existing enteral nutrition formulas (Table 8-1).
Enteral nutrition (EN) is clearly the preferred route of NI with limited roles for the use of TPN. EN provides benefits that are distinct from that of meeting caloric goals including preservation of gastrointestinal integrity and function and preservation of immunity. Starting EN early (as soon as the patient achieves hemodynamic stability) improves outcomes including gastrointestinal tolerance and may reduce mortality.
Prolonged starvation (particularly during the first week) builds a “caloric deficit” and may be detrimental. Total parenteral nutrition (TPN) supplementing EN is being tested as a means to overcome the caloric deficit and improve outcomes; the results of these trials are eagerly awaited. Attempts at minimizing starvation are essential for good outcomes.
The requirements of macro- and micronutrients such as certain amino acids, lipids, and vitamins are different in trauma and burn patients when compared to normal human beings or even to other critically ill patients including patients with sepsis.
The immune system plays an active role in the regulation of the availability of certain nutrients through active destruction or sequestration.
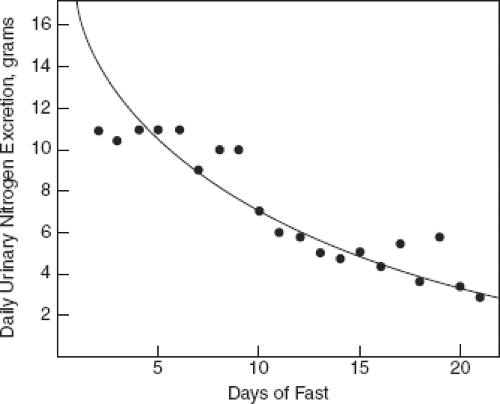 Figure 8-1. Daily urinary nitrogen excretion during prolonged fast. (From Freund E, Freund O. Beitrage zum Stoffwechsel im Hungerzustand. Med Klin 1901;15:69.) |
Table 8-1 Examples of Different Types of Enteral Nutrition Formulas. Nutrient (Expressed as % calories) from Two Different Companies in the United States | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II. Metabolic Demands of Trauma and Burns
The trauma patient exhibits significant metabolic changes that profoundly affect the adaptive response to starvation. Trauma patients become catabolic; the degree of catabolism is proportional to the degree of injury. Burn patients exhibit the highest degree of metabolic alterations among injured patients. Changes in burn and trauma may be unique; because of this, the principles that guide NI in other diseases may be unsuitable for the trauma patient.
Increased protein breakdown. Protein loss is augmented due to increased catabolism and loss through wounds following injury. Loss of protein can be high: Up to 15% of the lean body mass can be lost in 10 days. Severe protein malnutrition occurs when 25% to 30% of the lean body weight is lost. Thus, protein depletion (not calorie depletion) can become a life-threatening condition in severe trauma. The high catabolic rate is resistant to the provision of calories. However, protein synthetic rate does increase with amino acid infusions. Traditionally, traumatized patients should be offered 1.5 to 2 g/kg/day of protein.
Table 8-2 Formulas to Calculate Energy Expenditure
Harris–Benedict equation
EEE (males) = 66 + 13.7(wt in kg) + 5(ht in cm) − 6.8(age in y)
EEE (females) = 665 + 9.6(wt in kg) + 1.8(ht in cm) − 4.7(age in y)
Where EEE is estimated energy expenditure, wt is weight, and ht is height.
Hyperglycemia and resistance to insulin. Hyperglycemia is an independent predictor of poor prognosis with increased risk of infection, multiple organ dysfunction, and death. Hyperglycemia may be present in patients with no evidence of pre-existing diabetes. Hyperglycemia is due to complex factors related to stress. It is attributable primarily to excess hepatic gluconeogenesis due to the liver’s increased avidity for gluconeogenic substrates (i.e., lactate, pyruvate, and alanine). Hyperglycemia is also attributable to decreased glucose storage, increased circulating steroids, increased catecholamines, and increased glucagon.
Insulin release is suppressed within a few hours after trauma and restored in the later phase. However, hyperglycemia persists since insulin resistance is also characteristic of severe trauma.
Severe hyperglycemia is associated with decreased neutrophil chemotaxis, phagocytosis, oxidative burst, and superoxide production. Hyperglycemia can be worsened by inappropriate provision of glucose, a situation that can more easily occur with the provision of TPN or an attempt to deliver caloric goals beyond those needed by the patient.
Lipid mobilization. Mobilization of lipid stores (lipolysis) occurs after trauma. This is a result of the activation of triglyceride lipases by elevation in the levels of catecholamines, thyroid hormones, cortisol, adrenocorticotropic hormone (ACTH), glucagon, and growth hormones. Lipids remain the main energy source during recovery from trauma.
Resting energy expenditure (Table 8-2) is increased after trauma and is proportional to the degree of severity of injury. Observations of increased metabolic rates led to the misconception that provision of nutrients should be increased to meet metabolic demands (beyond that of 25 kcal/kg/day) and were traditionally known as “stress factors.” There is however, no evidence that increasing provision of nutrients beyond that of basic metabolic demands improves outcome, and indeed may worsen outcomes.
Vitamin deficiencies. There are no specific guidelines for the replacement of deficient vitamins beyond those advocated for normal individuals.
Immune-mediated amino acid destruction. Recent observations demonstrate that immune activation during trauma induces the release of arginase 1 contained in neutrophils and the accumulation of myeloid cells expressing this same enzyme occurs within hours of injury in immune tissues such as the spleen. Arginase 1 actively metabolizes arginine to ornithine and urea and can deplete this amino acid locally and systemically. Arginase 1 can also be released from injured hepatocytes and erythrocytes and is significantly abundant in packed red blood cells. Arginine is an essential amino acid for normal T lymphocyte function and nitric oxide production. Arginine depletion is now known to be associated with T lymphocyte dysfunction and decreased nitric oxide production in disease states associated with elevated arginase and may be a relevant problem in trauma.
III. Putting it all Together
Nutrition intervention is best done using published guidelines. Clinical management guidelines linked to performance-improvement processes are useful to prevent errors, initiate nutrition interventions, and guide safe therapy. Periodic updates of all protocols should be performed. Utilizing specialized nutrition teams and a registered dietitian skilled in NI are recommended.
Specific NI includes the following categories:
Oral intake at will
Controlled starvation
Enteral nutrition
Parenteral nutrition
Oral nutrition supplements
Inevitably, NI will fall into one or more of these categories. The option chosen will depend on a careful evaluation of the benefits and risks of a given choice, as well as a comparison with alternative interventional options. The physician should consider the following factors in determining how to make this decision.
Oral intake at will. Most adult human beings are able to maintain adequate nutrition intake, constantly meeting demands of water, electrolytes, vitamins, micro and macronutrients through volitional intake, and physiologic cues of thirst and hunger. Oral intake is the simplest and most natural way to provide adequate nutrition.
Full access? Get Clinical Tree






