KEY POINTS
Nutrients and gastrointestinal structure and function are linked to the pathophysiology of infection, organ dysfunction, and survival in critically ill patients.
Nutrition therapy may both positively and negatively influence the morbidity and mortality of critically ill patients.
When considering artificial nutrition in critically ill patients, enteral nutrition (EN) should be used in preference to parenteral nutrition (PN).
Strategies to optimize delivery of EN (eg, starting EN early, use of a feeding protocol with a high gastric residual volume threshold, use of prokinetic agents, and use of small bowel feeding) and minimize the risks of EN (eg, elevation of the head of the bed) should be considered.
For most patient populations in critical care in whom EN is not possible or feasible, the role of PN is controversial. Similarly, when to initiate supplemental PN when hypocaloric EN is not meeting the patient’s calorie or protein requirements is also controversial. Use of PN in these circumstances should be evaluated on a case-by-case basis taking into consideration the underlying nutrition risk of the patient.
Nutrition risk in the ICU can be identified by considering preexisting weight loss, decreased oral intake, prior stay in hospital before admission to ICU, preexisting comorbidities, and severity of current illness.
When PN is indicated, strategies that maximize the benefit (eg, supplementing with glutamine) and minimize the risks of PN (eg, hypocaloric dose, withholding soy-bean emulsion lipids, continued use of EN, and adequate glycemic control) should be considered.
Nutrition is considered an integral component of standard care in the critically ill patient. In humans, during stress associated with trauma, sepsis, or other critical illness, there is high consumption of various nutrients by the gastrointestinal tract, immune cells, kidneys, and other organs. Requirements for and losses of these nutrients may outstrip synthetic capacity, leading to an erosion of body stores and depletion of proteins and other key nutrients. Historically, in an attempt to mitigate such deficiencies and preserve lean body mass, traditional nutrition (protein, calories, vitamins, etc) has been provided to critically ill patients. The relative merits of nutrition were evaluated in the context of protein-calorie economy (weight gain, nitrogen balance, muscle mass and function, etc). In this chapter, we take a broader view of the benefits and risks of nutrition and we consider it as therapy that has the ability to modulate the underlying disease process, favorably alter immune responses, and impact outcomes of critically ill patients. The benefits of nutrition therapy in general include improved wound healing, a decreased catabolic response to injury, enhanced immune system function, improved GI structure and function, and improved clinical outcomes, including a reduction in complication rates and length of stay with accompanying cost savings.1 There are several studies that document that inadequate provision of nutrition to critically ill patients is associated with increased complications, prolonged length of stay in ICU and hospital, increased mortality, and increased health care costs.2-7 On the other hand, there are good data from large-scale observational studies8,9 and randomized trials10-12 that suggest better fed patients have better clinical and economic outcomes. Independent of their effects on nutritional status of the patients, key nutrients such as glutamine, arginine, and omega-3 fatty acids may also have direct effects on organ function and clinical outcomes of critically ill patients. Thus, nutrition therapy may be considered a specific therapeutic intervention by which the critically ill patient’s disease course may be altered, leading to a more favorable outcome.
There is considerable evidence linking nutrition (and lack thereof) and GI function to the pathogenesis of infection and organ failure in critical illness.13 Failure to obtain enteral access and to provide nutrients via the enteral route results in a proinflammatory state mediated by macrophages and monocytes. Oxidative stress is increased, severity of illness is exacerbated, and the likelihood of infectious morbidity, multiorgan failure, and prolonged length of stay is increased.14-16 In contrast, the provision of enteral nutrition results in higher levels of secretory IgA at mucosal surfaces throughout the body (lungs, lacrimal glands, tonsils, nares, and genitourinary system), greater preservation of gut-associated lymphoid tissue, and less intestinal permeability, all of which translates into improved clinical outcomes for critically ill patients.1
However, providing micro- and macronutrients is not without adverse effects or risks. Acquired infection, particularly ventilator-associated pneumonia (VAP), is a major problem for critically ill patients, resulting in increased morbidity, mortality, and health care costs.17,18 Pneumonia is likely due to aspiration of contaminated oropharyngeal/tracheal secretions and this is more likely to occur in a patient on EN, where EN promotes gastric colonization, gastroesophageal reflux, and pulmonary microaspiration. Parenteral nutrition has been associated with gut mucosal atrophy, overfeeding, hyperglycemia, adverse effects on immune function, an increased risk of infectious complications, and increased mortality in critically ill patients.19 While providing supplemental glutamine to seriously stressed critically ill patients may increase their chances of survival,20 depending on the circumstances, providing arginine to the same patients may increase their mortality.21 Therefore, nutrition therapy must be viewed as a double-edged sword, and strategies that maximize the benefits of nutrition support while minimizing the associated risks need to be considered in formulating clinical recommendations.
In developing such recommendations, the patient populations to which these recommendations will be applied must also be considered. Studies of nutrition in noncritically ill patient populations may not be generalizable to critically ill patients. For example, the treatment effect of PN in elective surgery patients is significantly different than the treatment effect of PN in critically ill patients.19
Even within subpopulations of critically ill patients, differences in outcome between the two routes of providing nutrition support are more likely to be seen with greater severity of illness. For example, the correlation between the importance of maintaining gut integrity and greater disease severity was demonstrated by a study evaluating septic complications in trauma patients, randomized at the time of surgery, to PN or to enteral tube feeding.22 In patients with high Abdominal Trauma Index (ATI) scores (>24), the incidence of septic complications was greater in the PN group than the group on enteral tube feeding (47.6% vs 11.1%, p <0.05). For those patients with moderate illness and lower ATI scores (<24), there was no significant difference in the incidence of septic complications between the parenteral and enteral groups (29.2% vs 20.8%, p = NS).22 Furthermore, in studies of EN versus PN in acute pancreatitis, faster resolution of the inflammatory response and significant differences in clinical outcomes (reduced septic morbidity and overall complications in the EN group) were seen in studies in which there were more patients with severe pancreatitis compared to studies with a higher proportion of patients with mild to moderate pancreatitis.23-25
In this chapter, we will discuss the relationships among nutrition, GI structure and function, immune function, and outcomes in critical illness. Upon this theoretical foundation, we will propose recommendations favoring the use of enteral nutrition over parenteral nutrition. Regardless of the route of artificial nutrition, we will suggest strategies that maximize the benefits and minimize the risks of both PN and EN.
RELATIONSHIP OF THE GASTROINTESTINAL TRACT, IMMUNE SYSTEM, AND ISCHEMIA/REPERFUSION INJURY
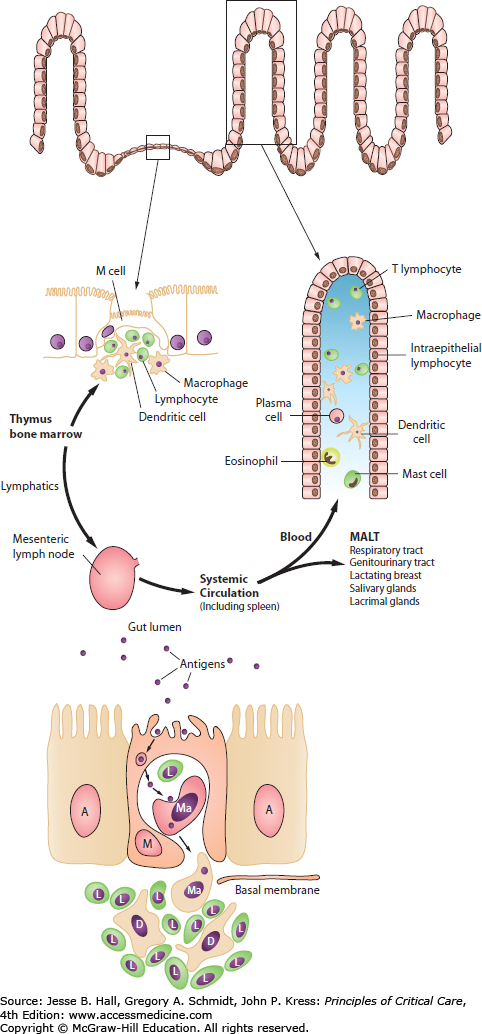
The GI tract is the largest immune organ in the body, containing 65% of immune tissue overall and up to 80% of the immunoglobulin-producing tissues of the body.15,16 In the fed state, the normal motility, villous microanatomy, rich blood supply, and epithelial intercellular tight junctions contribute to the overall integrity and barrier function of the GI tract. In response to luminal nutrients, propulsive contractions assist in controlling the concentration of luminal bacteria, and the secretion of bile salts, mucus glycoproteins, and secretory IgA retard bacterial adhesion to gut epithelial cells and subsequent translocation.26,27 The healthy gut acts as an important antigen-sensing organ, in which bacterial antigen is sampled and processed by the M cells, ultimately stimulating the release and maturation of a population of pluripotential stem cells or naïve CD4 helper T lymphocytes.28,29 These cells migrate out from the lamina propria of the gut, through the mesenteric lymph nodes and thoracic duct, and into the systemic circulation as a mature line of B- and T-cell lymphocytes. A proportion of these cells generated in the maturation of the pluripotential stem cells migrate out as mucosal-associated lymphoid tissue (MALT) to distant sites such as the lungs, genitourinary tract, breast, and lacrimal glands.26-29 Those that return to the Peyer patches of the enteric mucosa are known as gut-associated lymphoid tissue (GALT).27-29 In some situations, instead of seeing an increase in aspiration pneumonia in response to enteral feeding of critically ill patients, clinicians may instead see a reduced incidence of pneumonia22 due to maintenance of MALT in the lung by the trophic effects of luminal nutrients on the intestinal immune components.27-29
The intestinal microbiota and the function and structure of the GI tract are altered by changes brought on by critical illness. In the setting of increasing oxidative stress, where the pH or [Math Processing Error] levels within the lumen of the gut may drop, pathogenic bacteria like Pseudomonas and staphylococci undergo quorum sensing. If the number of organisms is high enough, these pathogenic bacteria express virulent genes, which allows adherence to the intestinal surface and a contact-dependent activation of the intestinal epithelial cell. A cytokine storm results with the release of inflammatory agents (interleukin-1, interleukin-8, and tumor necrosis factor) into lymphatic channels. A gut-lung conduit of inflammation results, as these cytokines pass through lymphatic channels and mesenteric lymph nodes into the thoracic duct and ultimately into the systemic circulation via the left subclavian vein. These proinflammatory cytokines pass directly into the microcapillary system of the lungs where activation of platelet activating factor and neutrophils lead to acute respiratory distress syndrome.
In a situation of even brief disuse, gut integrity may deteriorate. The mass of GALT and MALT tissue may diminish rapidly over a brief period of 7 to 10 days. Increased permeability occurs, opening up paracellular channels, allowing bacteria or other gut-derived factors such as endotoxin to activate elements of the innate immune system (macrophages).26 Activated macrophages will prime neutrophils passing through the splanchnic circulation. Primed neutrophils passing out to distant sites such as the liver, lung, and kidney may become activated by a second insult (such as hypoxemia or hypotension). At such sites, they may mediate tissue injury, resulting in the generation of oxidative species. Macrophage and subsequent neutrophil activation is a key step linking gut functional compromise with more systemic factors that adversely affect patient outcome.30 Activated macrophages and neutrophils also initiate the arachidonic acid cascade. Generation of prostaglandin E2 (PGE2) suppresses delayed hypersensitivity reaction, generates superoxide radicals, and leads to an increased susceptibility to sepsis. Generation of leukotriene B4 (LTB4) leads to chemotaxis and edema and the systemic inflammatory response syndrome (SIRS). Thromboxane A2, another product of this cascade, leads to vasoconstriction and thrombosis. This event, in turn, promotes physiologic shunts and multiple organ failure.31
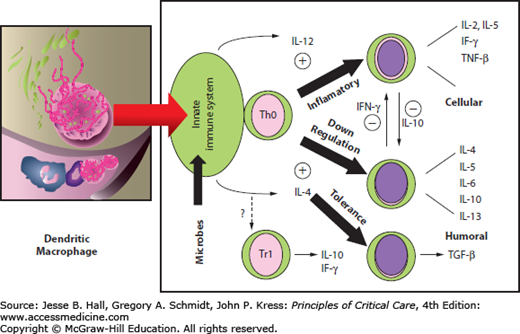
The overall tone of the systemic immune response may be modulated at the level of the gut. The dendritic macrophage cells act as an antigen-presenting cell (APC), which releases cytokines and activates the naïve CD4 T cells (Th0). The specific cytokines that are generated ultimately affect the differentiation pathway of these lymphocytes32 (Fig. 20-1). With gut disuse and fasting in critical illness, contractility is decreased, the hostile environment (low pH or [Math Processing Error] levels) suppresses growth of commensal organisms, and overgrowth of pathogenic bacteria occurs. These changes together with the absence of food antigen cause the dendritic cell (which has been sampling the luminal contents) to release interleukin-12 (Fig. 20-2). This cytokine causes naïve CD4 helper lymphocytes within the lamina propria to differentiate into a Th1 proinflammatory subset. This Th1 response results in the further release of other inflammatory cytokines, such as interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). Feeding supports the presence and role of commensal bacteria. In a fed state with food antigen present and a normal number of commensal bacteria, the dendritic cell releases interleukin-4 (IL-4). The production of IL-4 stimulates a change in naïve T cells (Th0) into the Th2 subset.32 Differentiation into Th2 lymphocytes causes further release of IL-4, interleukin-6 (IL-6), and interleukin-10 (IL-10). The Th2 response tends to oppose or attenuate the Th1 inflammatory response. Feeding is also associated with oral tolerance, which represents a Th3 subset of CD4 lymphocytes and is generated in the presence of IL-4, IL-10, and transforming growth factor-β (TGF-β), all of which tend to have immunosuppressive effects.32
THE IMPORTANCE OF MAINTAINING GASTROINTESTINAL INTEGRITY
The increase in gut permeability, which in some patients occurs over a very short period of time, has clinically important consequences for sick, critically ill patients. With loss of functional integrity, the tight junctions between the intestinal epithelial cells open up, the gut becomes “leaky,” and the patient experiences systemic bacterial challenge (through release of endotoxin and other gut-derived factors) and an exaggerated stress response with increased severity of disease.26 In a prospective randomized trial, Windsor and colleagues showed that patients with pancreatitis maintained on enteral tube feeding had no change in IgM antibodies to endotoxin over a week of enteral feeding.24 In contrast, controls placed on PN and gut disuse demonstrated a significant increase in IgM antibodies to endotoxin of 25% in response to a week of parenteral feeding (p < 0.05).24 In a second study, increased gut permeability (measured by enteric absorption and urinary excretion of polyethylene glycol) and systemic endotoxemia correlated significantly with greater disease severity in patients with acute pancreatitis.33 In a prospective randomized trial, normal healthy volunteers randomized to PN and gut disuse for 7 days demonstrated an exaggerated stress response to a standard IV challenge of E. coli endotoxin, as evidenced by higher glucagon, epinephrine, tumor necrosis factor, and C-reactive protein (CRP) levels and greater muscle catabolism compared to a study group receiving a week of enteral feeding.34 In two studies in patients with acute pancreatitis, significantly faster resolution of the SIRS response and “resolution of the disease process” (resolution of pain, decreasing amylase, and successful advancement to oral diet) was seen in patients randomized to EN compared to those placed on PN.24,35
Consistent with the theoretical evidence presented, there are 13 studies of critically ill patients with surgery, trauma, and medical illnesses that evaluated the benefits of EN compared to PN. Compared to PN, EN was associated with a significant reduction in infectious complications (RR 0.62; 95% confidence intervals [CIs] 0.62, 0.84; p = 0.002).36 No significant differences were seen in mortality between groups. Thus, in general, by feeding via the enteral route, we can expect to reduce the infectious complications associated with nutrition therapy in critically ill patients without adversely affecting survival.
At the bedside, clinicians fail to recognize the relationship between gut structure and function and adverse patient outcome, primarily because there is significant delay in the development of complications that arise from poor management decisions related to enteral therapy. If mistakes are made with oxygen delivery, hypoxemia ensues immediately and the patient may deteriorate within minutes. If mistakes are made with volume resuscitation, there is a degree of delay, and problems arising from decreased vascular volume, hypoperfusion, and increasing azotemia may not develop for 12 to 24 hours. If no effort is made to maintain gut integrity, the complications that arise as a result may not develop for 3 to 5 days. At that point, when nosocomial infections occur or organs begin to fail, the clinician does not connect the development of these complications with management decisions made 5 days before with regard to enteral nutrition. In fact, only in prospective randomized trials can it be determined that had gut integrity been maintained, there might have been a decrease in the number of nosocomial infections, the number of organs failing, and the overall length of stay in the ICU prior to discharge.
NUTRITIONAL SCREENING and ASSESSMENT
Nutrition screening at admission is essential to the identification of patients who are at risk of adverse events due to their nutritional status and is recommended by various organizations.37-39 Various screening tools currently exist for use in hospitalized patients and are based on criteria such as history of unplanned weight loss and decreased oral intake, body mass index, acute illness/severity of disease/gastrointestinal symptoms, mobility, and physical assessment.40-45 None of the screening tools have been developed or validated specifically for the critically ill population, in whom acute inflammatory responses have a rapid, catabolic effect on lean body mass resulting in poor nutritional status at ICU admission.
There is strong observational evidence to show that not all critically ill patients respond to artificial nutrition the same way. In a recently published prospective study of 2772 mechanically ventilated adult patients from 167 ICUs around the world, the association of nutritional adequacy and clinical outcomes was examined.9 Data were collected for maximum of 12 days and regression models were developed to explore the relationship between nutrition received and 60-day mortality. The results suggested that an increase of 1000 calories per day was associated with an overall reduction in mortality (odds ratio for 60-day mortality 0.76; 95% CIs 0.61-0.95; p = 0.014). Interestingly, in a subgroup analysis, the beneficial treatment effect of increased calories and protein on mortality was observed mostly in patients with a BMI <25 and >35 with less benefit for patients in the BMI 25 to 35 group. These data support the notion that artificial nutrition may exert a differential treatment effect with respect to mortality in different subgroups of ICU patients, thereby making it difficult to accurately assess nutritional needs even within the same setting. Practitioners need to discriminate which subgroups might benefit the most (or least) from nutrition.
A novel approach to quantifying risk in the critically ill patient is therefore warranted, especially one that accounts for inflammation as well as acute and chronic starvation. Consistent with groundbreaking definitions of malnutrition by Jensen and colleagues,46 the NUTrition Risk in the Critically ill score (NUTRIC score) was developed, in which risks of adverse events that may be modifiable by nutrition therapy were quantified.47 In a secondary analysis of a prospective observational study, data for key variables considered for inclusion in the score were collected in 598 critically ill patients. Variables included age, baseline APACHE II, baseline SOFA score, number of comorbidities, days from hospital admission to ICU admission, Body Mass Index (BMI) <20, estimated percent of baseline oral intake in the week prior, weight loss in the last 3 months and serum IL-6, procalcitonin (PCT), and CRP levels. After multivariable modeling, the final NUTRIC score consisted of six variables, that is, age, baseline APACHE II, baseline SOFA score, number of comorbidities, days from hospital admission to ICU admission, and serum IL-6 and was found to be highly predictive of outcomes such as mortality and duration of mechanical ventilation. Patients with higher NUTRIC scores had worse outcomes compared to those with lower scores. More importantly, patients with a higher NUTRIC score were found to benefit the most from meeting their estimated nutrition needs compared to patients with a lower NUTRIC score who did not have any benefit from more nutrition. This novel scoring tool will help practitioners identify which critically ill patients are more likely to benefit from aggressive nutrition.
Determination of caloric requirements is very important on the initial nutritional evaluation, helping set the goal (number of required calories) of nutritional therapy. Caloric requirements are best determined using simple equations (25-30 kcal/kg per day) or by specific measurement via indirect calorimetry. There is no strong evidence to suggest one method of determining protein-energy requirements is better than the other. What is more important is that once those targets are set, that efforts to achieve them as soon as possible are made.
Unfortunately, obtaining enteral access early in the course of the critical illness may be very difficult. With greater severity of illness, patients become more prone to ileus with gastroparesis, high residual volumes, and intolerance of gastric feeds. Early on, the hypermetabolic response, SIRS, high doses of narcotic analgesics, and electrolyte abnormalities may potentiate gastroparesis. Compounding the problem is the fact that disuse of the gut reduces the secretion of prokinetic hormones such as gastrin, bombesin, and motilin.26,27
The ability to obtain enteral access may be vital to the success of nutritional therapy in the critically ill patient. Each institution needs specialists who have the skills to place tubes at the appropriate levels of the GI tract, with techniques that can usually be done at the bedside with minimal or no sedation. A number of newer tubes and techniques have been described for blind postpyloric placement at the bedside, which in the hands of a dedicated nurse, dietitian, or intensivist should be successful in >85% of cases.48-51 Newer guidance systems using magnets on the tip of the feeding tube (guided by handheld magnets on the outside), tracking systems with a GPS device in the tip of the tube (visualized by a monitor on the outside), and optical guidance systems using fiberoptic strands or the CMOS camera chip from cell phones placed within the feeding tube, all should serve to enhance the safety and success rate of bedside placement. In cases where bedside placement is unsuccessful or deeper jejunal placement is required (such as in patients with severe acute pancreatitis), enteral access to the small bowel may require endoscopic or fluoroscopic placement. For these latter patients, transport out of the ICU should be avoided to prevent an increased risk of mishaps (eg, cardiopulmonary arrest, new dysrhythmias, or loss of central IV line access) and pulmonary aspiration.52-54
Physical examination by the clinical nutritionist may be the most important element of monitoring the patient on enteral tube feeding. Abnormalities on physical examination usually reflect segmental abnormalities in contractility of the GI tract. Bloating, abdominal distention, hyperresonance, and increased residual volumes may signify delayed gastric emptying. In patients placed on nasogastric drainage, output of >1200 mL/d may indicate relative gastroparesis. Contractility of the colon may be assessed by passage of stool and gas. The presence of bowel sounds is a poor indicator of contractility in the small bowel, as evidenced by the fact that nasogastric suction will reduce its detection. Studies performed on the postoperative return of bowel function or contractility have provided valuable findings for the clinician. Invariably, contractility in the stomach stops initially, followed next by colonic contractility. Small bowel function or contractility appears to be retained the longest.55 In most critically ill patients (particularly patients with trauma), who on baseline evaluation have grossly abnormal physical examinations, tolerance to enteral tube feeding may be defined by slight decreases in abdominal distention and abdominal discomfort in the absence of high gastric residual volumes, metabolic acidosis, third-spacing of fluids, or a worsening clinical condition. These findings on serial physical examinations determine whether the position of the feeding tube needs to be changed (ie, placing the tip of the tube lower down in the GI tract at or below the ligament of Treitz), whether a tube with simultaneous aspirating and feeding capabilities needs to be added, or whether the feeds need to be temporarily discontinued.
STRATEGIES TO MAXIMIZE THE BENEFITS AND MINIMIZE THE RISKS OF ENTERAL NUTRITION
While enteral feeding is the preferred route of nutrient administration, how soon it should be started after an acute injury or insult is not clear. In critically ill patients, there were 14 randomized controlled trials comparing early EN (ie, that started within 24-48 hours of admission to the ICU) to some form of delayed nutrient intake (ie, delayed EN or oral diet).56 When results from these studies were aggregated, early EN was associated with a trend toward a reduction in mortality (RR 0.60; 95% CIs 0.46, 1.01; p = 0.06) when compared to delayed nutrient intake. Seven studies reported infectious complications.57-63 When these were aggregated, early EN was associated with a significant reduction in infectious complications (RR 0.76; 95% CIs 0.59, 0.98; p = 0.04) when compared to delayed nutrient intake. No differences in length of stay were observed between groups. All 13 studies reported nutritional end points and showed a significant improvement in the groups receiving early EN (eg, improvements in calorie intake, protein intake, percentage of goal achieved, and better nitrogen balance achieved). There were no differences in other complications between the groups.
Although the results lack statistical significance, they do suggest a large improvement in clinical outcome and a significant increase in nutrient delivery associated with early enteral feeding. However, before endorsing the concept of early enteral feeding, one must consider the potential risks of such a strategy. Two recent nonrandomized studies suggest that early enteral feeds delivered into the stomach may be associated with increased complications.64,65 In contrast, Taylor and colleagues combined an aggressive early feeding protocol with the use of small bowel feedings and documented that head-injured patients fed aggressively, compared to standard (slower) provision of EN, not only had better nutritional status, but also had fewer complications and a more rapid recovery from their illness.10 Moreover, in a large multicenter observational study, Artinian and colleagues demonstrated that early EN (within 48 hours) was associated with a small increase in pneumonia rates but not withstanding, these patients who were fed early had a lower mortality rate compared to patients who received delayed EN.66
Synthesizing these discordant results, it would seem that early EN may be associated with improved clinical outcomes when done in such a way that maximizes the benefits and minimizes the risks (see below). Careful early EN, particularly if delivered distal in the small bowel, will reduce the risk of EN and provide the benefits of maintaining gastrointestinal structure and function. Furthermore, it should be noted that the goal of early EN, while critically ill patients are still early in the acute phase of their illness, is to provide enough critical nutrients to the gut to modulate the disease process and enhance gut barrier structure and function, not to meet their caloric requirements as soon as possible. Thus for some patients with evidence of inadequate oxygen delivery, specific nutrients (eg, glutamine and antioxidants) may be more important to provide in the first few days of critical illness. If patients are still on high-dose inotropes to maintain adequate blood pressure, the risk of providing EN may outweigh the benefits. However, recent data suggest that even patients on vasopressors may benefit from early EN. Khalid and colleagues used a multi-institutional database to identify mechanically ventilated patients on vasopressors and compared the outcomes of those who received early EN to those were received delayed EN, using sophisticated propensity matching analysis to adjust for confounding variables.67 They demonstrated that the group of patients that received early EN had a much lower mortality rate than those that received delayed EN. Moreover, they described that the sickest patients, those on multiple vasopressors experienced the largest benefit. This is the strongest available evidence to support the safety and efficacy of feeding the hemodynamically challenged patient. By no means are we advocating that EN has any role in the unresuscitated, unstable patient. But once fully resuscitated, despite the presence of vasopressors, EN should be initiated. If there are concerns about tolerating high-volume intragastric nutrition in such patients, either direct jejunal feeding or initiating low-volume feeds (trophic feeds) at 10 to 20 mL/h for 24 hours then reassessing could be considered.
It is important on initial evaluation to assess the patient’s risk for aspiration on EN. Aspiration may occur from the antegrade passage of contaminated oropharyngeal secretions or the retrograde passage of contaminated gastric contents into the larynx. Regurgitation occurs more frequently than aspiration.68 A number of risk factors have been identified that increase risk of aspiration in the ICU.69 While it is difficult to quantify or stratify degree of risk among these factors, a simple categorization differentiates major risk factors for which change in management strategy may be needed, versus additional minor risk factors that may not warrant specific change in therapeutic course. Major risk factors include documented previous episodes of aspiration, decreased level of consciousness (including sedation or increased intracranial pressure), neuromuscular disease, structural abnormalities of the aerodigestive tract, need for endotracheal intubation, overt vomiting or regurgitation, need for prolonged supine position, and persistently high gastric residual volumes.69Additional risk factors include presence of a nasoenteric tube, noncontinuous or bolus intermittent feeding, abdominal/thoracic surgery or trauma, delayed gastric emptying, poor oral care, advanced age, inadequate nursing staff, large bore feeding tube, malpositioned enteral tube (back into the esophagus), or transport out of the ICU.1,69 Strategies to prevent aspiration in patients receiving nutrition support who have significant risk factors, as outlined below, should be utilized to minimize the risks associated with EN in this setting.
A number of strategies may be employed to maximize the delivery of EN while minimizing the risks of gastric colonization, gastroesophageal regurgitation, and pulmonary aspiration (Table 20-1). By delivering enteral feeds into the small bowel, beyond the pylorus, the frequency of regurgitation and aspiration, and possibly the risk of pneumonia, is decreased while at the same time nutrient delivery is maximized.70 There are 11 randomized trials that evaluated the effect of route of feeding on rates of VAP.71 When these results were aggregated, there was a significant reduction in VAP associated with small bowel feedings (RR 0.77; 95% CIs 0.60, 1.00; p = 0.05) compared to gastric feeding. Therefore, the converse is also true. In some patients, intragastric feeding may be associated with inadequate delivery of nutrition, increased regurgitation, pulmonary aspiration, and pneumonia, particularly if patients are cared for in the supine position.
Summary of Strategies to Optimize the Benefits and Minimize the Risks of Enteral Nutrition and Total Parenteral Nutrition
| Enteral Nutrition | Total Parenteral Nutrition |
|---|---|
| Initiate early, within 24-48 hours of admission | Hypocaloric dose |
| Use small bowel feedings | Do not use lipids for short-term use (<10 days) |
| Elevate head of the bed | |
| Use motility agents | Tight control of blood sugars |
| Use feeding protocol that enables consistent evaluation of gastric residual volumes and specifies when feeds should be interrupted | Supplement with glutamine |
| Use concentrated feeding formulas in cases of intolerance | Continue to trickle concentrated amounts of enteral nutrition if able |
| Consider formulas with immune additives |
The clinical implications of these findings are influenced by the inherent difficulties in obtaining small bowel access. Given that some patients will tolerate intragastric feeds, it seems more prudent to reserve small bowel feeds for patients at high risk for intolerance to EN (due to use of inotropes, continuous infusion of sedatives, paralytic agents, high gastric residual volumes, or patients with high nasogastric drainage) or at high risk for regurgitation and aspiration (nursed in prolonged supine position).

Full access? Get Clinical Tree