Fig. 34.1
EsophyX® device. (Reprinted with permission of EndoGastric Solutions, Inc., Redmond, WA. Copyright © 2014 EndoGastric Solutions, Inc.)
TIF involves an endoscopically guided transoral insertion of the EsophyX® device to create a 270°, 2–3 cm esophagogastric valve by placing 12–20 full-thickness polypropylene H-fasteners. This approach augments the gastroesophageal flap valve, the valve visualized in the stomach which correlates to the angle of His on external view of the stomach. To undertake TIF, a flexible video endoscope is passed through the EsophyX® device, and both are passed transorally into the stomach. The entire procedure is undertaken with endoscopic guidance. Through the EsophyX® device a helical screw device is deployed and anchored into the deficient gastroesophageal flap valve and the screw is used to draw the deficient gastroesophageal valve into the device. The device is then closed and the gastric fundus and the deficient gastroesophageal valve is compressed allowing polypropylene H-fasteners to be passed across the esophagus and the gastric fundus, anchoring these structures together and augmenting the valve. A total of 12 H-fasteners are used to construct the fundoplication. The procedure may also result in reduction of all small hiatal hernias up to 2 cm in size [7]. Factors that affect positive outcomes after TIF include a large number of fasteners and hiatal hernia less than or equal to 2 cm. Presence of esophageal dysmotility and hiatal hernia may increase the risk of recurrence of GERD symptoms [8].
Longer-term data suggest 70–80 % of patients are completely off PPIs following TIF and only a small group of patients have to take PPIs daily, which would suggest the TIF has failed [9, 10]. Following TIF, DeMeester scores were reduced and normalized in 60–70 % of patients. The average number of acid reflux episodes per 24-h testing period was also significantly reduced following TIF and normalized in 80–90 % of patients [10]. Most patients who underwent TIF report amelioration of symptoms of heartburn and regurgitation. For patients with failed TIF, revisional laparoscopic fundoplication was found to be safe and efficacious resulting in objective reflux control [11]. Complications noted by various published series indicate hemorrhage as the most common complication; however, the risk is less than 1 % and amenable to endoscopic control of bleeding. There is a 0.7 % risk for esophageal perforation associated with TIF. TIF appears to consistently provide symptomatic relief with promising levels of patient satisfaction [11, 12].
In patients with erosive esophagitis or Barrett’s esophagus, TIF may not be considered adequate anti-reflux therapy, as normalization of esophageal pH is of utmost importance [10].
The Stretta System (Curon Medical, Fremont, CA)
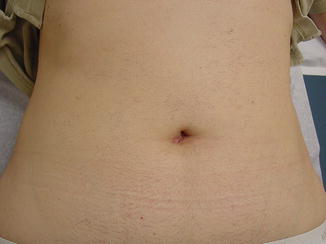
The Stretta system (Fig. 34.2a, b) is another endoscopic endoluminal system utilized for treating GERD. It has been available for more than a decade. This unique system applies radiofrequency ablation energy (RFA) to the muscularis propria in the LES without causing a significant thermal burn to the mucosa. This device utilizes an inflatable balloon through which four electrodes emanate; these electrodes deliver RFA energy under a controlled temperature to cause inflammation and healing by fibrosis. Stretta works by reducing the relaxation pressure of the LES, reducing tissue compliance without fibrosis, and increasing the smooth muscle fiber size with more muscle fibers per muscle bundle resulting in lengthening and thickening of the sphincter, thereby improving the barrier function of the LES mechanism [13]. To minimize collateral injury, the device utilizes continuous saline irrigation while continuously monitoring the temperature of the mucosa and muscularis propria.
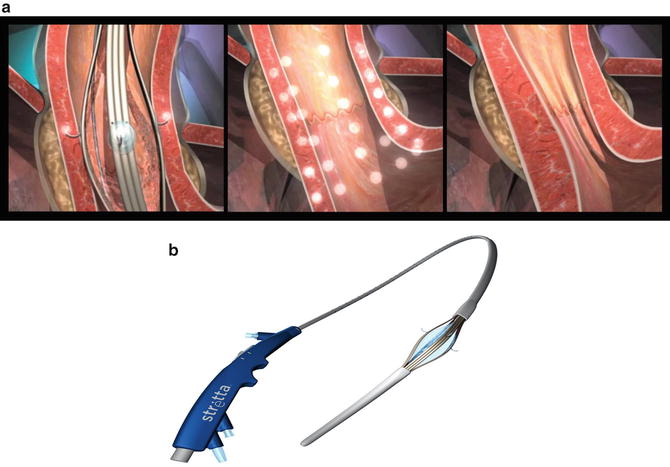

Fig. 34.2
(a, b) Stretta device. (Reprinted with permission of Mederi Therapeutics, Norwalk, CT. Copyright © 2014 Mederi Therapeutics, Inc.)
Although the Stretta procedure has shown promise in the ability to improve heartburn and decrease PPI usage over a 12 months period and up to 5 years after application, only a small number of studies have long-term outcomes [7, 14, 15]. Abdel Aziz et al. reported through a prospective randomized trial that the total esophageal acid exposure was reduced after the Stretta procedure and this effect was sustained through the 12 months of follow-up [16]. The Stretta system alleviated GERD symptoms, including heartburn, and reduced PPI requirements in nearly two-thirds of patients. Reymunde and Santiago reported a 4-year follow-up with sustained improvements in symptoms, quality of life, and PPI use after Stretta [17]. The efficacy was again demonstrated by Dughera et al. as they documented that 72.3 % of patients with GERD remained PPI-free at 48 months [18]. A meta-analysis of 18 studies reporting data on 1,441 patients was reported by Perry et al. [19]. This meta-analysis showed significant improvement in symptoms of reflux, improved quality of life measured by GERD QOL scale, and significantly reduced DeMeester scores. While pre-procedure DeMeester scores were reduced from 44.4 to 28.5, they did not normalize (normal being <14.7).
In four randomized controlled trials comparing sham Stretta or PPI versus Stretta alone, patients who underwent Stretta had significantly reduced GERD health related quality of life complications, reduced use of PPIs, reduced esophageal acid exposure, normalization of LES pressures, and improved grades of esophagitis [15, 16, 20, 21]. Noar et al. prospectively followed 217 patients with 10-year follow-up after Stretta. Interestingly, the most common short-term side effect reported was chest pain. Seventy-two percent of patients reported normalization of GERD-HRQL and more than 50 % reported reduction in PPI use with 41 % of patients completely off of PPIs. In this series, no esophageal perforations were noted; other series have reported esophageal perforation as a dreaded, albeit very infrequent, complication [22]. No data is available for the use of Stretta in patients with severe esophagitis, hiatal hernias more than 2 cm (this is a contraindication to the procedure), Barrett’s esophagus, or with a history of autoimmune disease, collagen vascular disease, and/or coagulation disorders. Based on the available literature, Stretta can be recommended for patients with acid reflux, but without hiatal hernia more than 2 cm. Stretta seems best applied to patients with excess gastroesophageal reflux who have had operations which have reduced gastric volume, such as sleeve gastrectomy or gastric bypass for weight loss.
The LINX® Reflux Management System (Torax Medical, Inc., Shoreview, MN)
In 2012, the FDA approved the LINX® system (Fig. 34.3) for the treatment of GERD. The LINX® system consists of a small band with interlinked titanium beads with magnetic cores. Utilizing laparoscopy, the band is placed around the GE junction in such a way that the magnetic attraction between the beads works to keep the GE junction closed at rest. With swallowing, as the food bolus reaches the LES, esophageal generated swallowing forces push the food and liquid through the GE junction as the magnetic beads separate. After the food bolus passes the GE junction, the magnetic force causes the LES to close immediately preventing acid reflux.
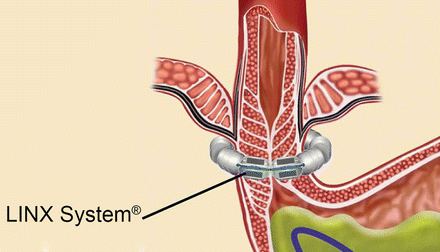

Fig. 34.3
The LINX® system. (Reprinted with permission of Torax Medical, Inc., Shoreview, MN)
In a feasibility trial, 44 patients with GERD were enrolled between 2008 and 2009 and 2-year follow-up results were reported [23]. At 1 and 2 years, 77 and 90 % of patients had normal esophageal acid exposure. The trial documented that the mean percentage of time with a pH less than 4 decreased from a baseline of 11.9–3.1 % (p < 0.0001) at 1 year and to 2.4 % (p < 0.0001) at 2 years. Patient satisfaction was 87 % at 1 year and 86 % at 2 years. Based in part on this data, the FDA approved the use of the LINX® system for the treatment of GERD. Patients excluded from this trial had a hiatal hernia more than 3 cm, dysphagia, greater than grade A esophagitis, and Barrett’s esophagus. In 2013, Bonavina et al. reported a prospective case series of 100 consecutive patients, who underwent laparoscopic LINX® placement. With median implant duration of 3 years, this series reported improved total acid exposure time and improved GERD HRQL scores with no long-term complications such as device migration or erosion. However, three patient had the device removed for persistent dysphagia or GERD [24].
The LINX® system is a new approach for treating acid reflux. Long-term data about efficacy and complications are lacking. Therefore, the LINX® reflux management system can only be recommended with caution as a first-line therapy in treating GERD.
EndoStim (EndoStim®, St. Louis, Missouri, and The Hague, The Netherlands)
The EndoStim system is a new device focusing on increasing lower esophageal sphincter tone by delivering electric stimulation to the lower esophagus. This system is proposed as a long-term treatment option for patients with severe GERD.
EndoStim’s Stimulation System involves laparoscopic placement of electrodes on the lower esophagus which are connected to an electrical simulator placed in the subcutaneous pocket made in the abdominal wall. It delivers low-energy electrical pulses to the gastroesophageal junction. Rodriguez et al. [25] reported 2 years outcome of electrical stimulation therapy in 25 patients. They reported significant and sustained improvement in symptoms and reduction in PPI use. This group reported improved GERD Health Related Quality of Life improvement off PPI. Possible complications of the EndoStim system include esophageal perforation during the placement of the electrodes which may lead to removal of the device.
The EndoStim is a new system, and the data are evolving. Due to the paucity of convincing data in long-term treatment of acid reflux, the EndoStim system is not approved for use in the USA by the FDA.
Laparo-Endoscopic Single Site (LESS) Fundoplication
The long-term outcomes associated with laparoscopic fundoplication for treating GERD are well documented in the literature. Its mention in any discussion about efficacious therapy for GERD is warranted given its proven safety and efficacy. Laparoscopic fundoplication is the “first-line” surgical therapy for treating GERD.
With laparoscopy, operative techniques and technology are continuously evolving. The evolution of conventional laparoscopy to LESS surgery began with the observation that a laparoscopic cholecystectomy could be safely undertaken with fewer port sites and trocars [26]. The most recent innovative laparoscopic approach for treating GERD has been the Laparo-Endoscopic Single Site (LESS) fundoplication. This operation is undertaken through a single 8–12 mm incision at the umbilicus, preserving the umbilical ring [27] (Fig. 34.4). The fundoplication constructed and the conduct of the operation in general are not different than when laparoscopic fundoplication is undertaken. Because the approach is “scarless,” LESS surgery is a cosmetically superior approach to treating gastroesophageal reflux compared to multi-incision laparoscopic surgery, which usually involves five or more ports each through its own incision to complete. The “scar” with LESS surgery is hidden at the umbilicus, itself a scar; therein the operative approach is “scarless” (Fig. 34.5). As well, this single incision laparoscopic may lead to reduced postoperative pain, a reduced incidence of wound infections and complications, and a shorter length of stay.

Fig. 34.4
Port placement for laparoendoscopic single site fundoplication