Fig. 13.1
Noncontrast CT scan of acute subdural hematoma
Mortality
Mortality in aSDH is high, ranging from 40 to 60 %. Underlying brain injury resulting from aSDH is the significant cause of the high mortality. Higher mortality is seen in older patients and patients on anticoagulants with mortality rates of 60 % and 90–100 % respectively. Factors influencing mortality include mechanism of injury, age, neurologic condition on admission, and postoperative ICP [2].
Surgical Treatment
Indication
Patients with aSDH thickness >10 mm or midline shift (MLS) >5 mm often receive surgery. If patients have aSDH thickness <10 mm and MLS <5 mm, surgery may be indicated if patients have GCS drop of two or more points from the time of injury to admission, asymmetric or fixed and dilated pupils, and/or intracranial pressure >20 mmHg2.
Standard of Care
Common surgical techniques used in treatment of aSDH are craniotomy, burr hole, twist drill, subtemporal decompressive craniectomy, and large decompressive hemicraniectomy. Choices of surgical techniques vary depending on situation and surgeon’s training. One study comparing the efficacy of surgical techniques used for aSDH showed significantly increased mortality and reduced functional recovery rate for patients with GCS scores between 4 and 6 when burr hole trephination was used compared with craniotomy. Another study compared craniotomy, craniotomy with dural grafting, and decompressive craniectomy (DC) in 113 patients and found that all 17 patients undergoing DC died with no other significant differences found between treatment groups [1].
Latest Advances
The clinical cost-effectiveness of DC has been an important issue. The advantage of DC is that it allows more effective control of high ICP which is critical in determining outcome after traumatic brain injury. The disadvantage of DC is that it requires a cranioplasty procedure with its complications and that not all patients have brain swelling and do not benefit from the increased management of ICP provided by DC. Recent study showed that out of 91 patients, of whom 51 underwent DC and 40 underwent craniotomy, the overall mortalities of DC and craniotomy were 38 % and 32 % respectively. This showed that the unadjusted outcome of craniotomy and DC were not significantly different, even though there were higher proportion of major extracranial injury in patients undergoing DC. This suggests that DC may be more effective in selected patients as mortality rates did not significantly differ even with increased initial injury severity in patients who underwent DC [7].
Nonsurgical Treatment
Indication
Neurologically stable patients with aSDH <10 mm and MLS <5 mm with normal pupillary movements and no intracranial hypertension should be considered for nonsurgical management. Patients with a stable clinical condition and normal ICP are at lower risk than unstable patients with elevated ICP [8].
Standard of Care
Patients should be closely observed for neurological deterioration which may necessitate evacuation. Patients with traumatic brain injury often undergo serial follow-up head CT scans during the first 36 h post injury because of the high probability of clot expansion during this time [8].
Latest Advances
Hypertonic/hyperoncotic treatment (HHT) improves functional and histologic outcome after aSDH through decreasing ICP. HHT restores microcirculation through improved rheology. In addition, endothelial cell swelling is reduced, capillary diameters increased, and leukocyte endothelium interactions are reduced which all aid in reduction of ICP [9]. Recent study by Rahimi et al. has shown that recombinant human erythropoietin (EPO) applied locally as direct cortical application in rats can show similar neuroprotective events through potential mechanisms including anti-apoptosis, anti-inflammation, and anti-oxidation. EPO, in combination with hematoma evacuation, was shown to decrease post-ASDH lesion volume by 68 % [10].
Chronic Subdural Hematoma
Overview
Incidence
Chronic subdural hematoma (cSDH) mainly occurs in older patients. The incidence is 3.4 per 100,000 patients and 8.2 per 100,000 patients per year for adults younger than 65 and older than 65 years respectively [11]. Risk factors include atrophy, alcohol abuse, seizures, cerebrospinal fluid diverting shunts, coagulopathies, and patients at risk for falls [2].
Pathophysiology
Some cSDHs may start as acute subdurals triggering an inflammatory response. This results in fibroblast invasion into the clot and formation of neomembranes in both the cortical and dural surfaces. Ingrowth of neocapillaries, enzymatic fibrinolysis, and liquefaction of blood clot follow and the fibrin degradation products enter new clots to prevent hemostasis, resulting in cSDH [2].
Clinical Presentation
Common presenting symptoms include gait disturbance, hemiparesis, headaches, dementia, incontinence, and consciousness disturbance. In a study with 500 consecutive cases of cSDH patients, gait disturbance and hemiparesis were present in 63.0 and 58.6 % of the patients [2]. As many as half of patients may not recall significant traumatic events. Figure 13.2 shows a CT scan of patient with cSDH.
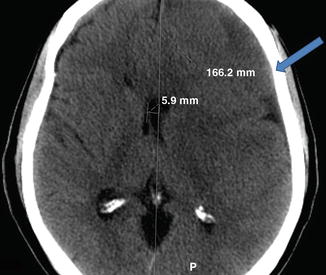

Fig. 13.2
Noncontrast CT scan of left frontoparietal chronic subdural hematoma with right midline shift
Mortality
Mortality of cSDH patients is high with perioperative mortality ranging from 1.2 to 11 % and the 1 year mortality of elderly patients treated with drainage intervention around 32 %. One study shows that out of 209 post-cSDH patients, the mean survival rate was 4.4 years which was significantly shorter than the mean survival of 6.0 years for an age matched non-cSDH group computed from actuarial life-tables [12].
Surgical Treatment
Indication
Surgical treatment is indicated in patients with symptomatic lesions including focal deficit and mental status changes. In addition, subdurals with maximum thickness >1 cm are often evacuated [2].
Standard of Care
Current surgical treatment options include craniotomy, burr holes, twist drill, and subdural evacuating port system (SEPS). No consensus exists as to which surgical method is the best to treat cSDH. Burr hole craniotomy is the most popular procedure for primary cSDH and can be done under general or local anesthesia [13]. Twist drill craniotomy decompresses the brain more slowly and avoids rapid pressure shifts which may be correlated with intracerebral hemorrhage complications. In addition, twist drill craniotomy can be performed at the bedside. SEPS is a variant of twist drill craniotomy with a hermetically sealed closed drainage which reduces infection rate [14].
Latest Advances
Twist drill craniostomy (TDC), although time-saving and cost-effective, has complications that include inadequate drainage, brain penetration, acute epidural hematoma and catheter folding. A modified technique of TDC has been developed to overcome these complications. From July 1992 to December 2011, 50 cSDH patients have been treated with modified TDC and no mortality related to the procedure was observed with 6 % mortality rate due to poor pre operative neurological status [15].
Nonsurgical Treatment
Indication
Patients without neurologic deficits are often managed without drainage.
Standard of Care
cSDH patients can be treated with seizure prophylactic anticonvulsants as seizure frequency in these patients range from 2 to 19 %. Conflicting viewpoints on risk and benefit of using anticonvulsants exist. Corticosteroids have also been used to treat cSDH with the rationale that anti inflammatory action will prevent hematoma and maintenance [16]. ACE inhibitors, which have antiangiogenic effects, have also been shown to reduce recurrence of cSDH. One study showed that out of 81 cSDH patients treated with ACE inhibitors, only 5 % showed recurrence of cSDH as compared to 18 % recurrence rate of patients who did not receive ACE inhibitors [17].
Latest Advances
Hyperfibrinolytic activity has been shown to be critical for liquefaction of hematoma and progression of cSDH. In addition, increased permeability of capillaries in hematoma leads to enlargement of cSDH. Plasmin acts on both fibrinolytic and kalikrein systems; thus targeting plasmin may ameliorate the progression of cSDH. Recently, tranexamic acid, an antifibrinolytic drug which inhibits plasminogen activation and plasmin activity, has been used to treat cSDH patients. Studies show that out of 18 cSDH patients treated with tranexamic acid only, clinical symptoms improved and hematomas were fully reduced in all patients. No recurrence or thromboembolic side effects have been observed. In addition, side effects of tranexamic acid are mild and uncommon with thromboembolic cerebrovascular events not occurring significantly more than control group. Tranexamic acid may be an effective medical therapy for cSDH [18].
Traumatic Parenchymal Lesions
Overview
Incidence
Traumatic parenchymal lesions comprise 8.2 % of all TBI, 13–35 % of severe TBI, and up to 20 % of operative intracranial lesions in representative series [1].
Classification
Traumatic parenchymal lesions are heterogeneous in nature, and these lesions have been divided into two broad classes: focal and nonfocal lesions. Focal lesions include intracerebral hematoma, delayed traumatic intracerebral hematoma, contusions and infarctions. Nonfocal lesions are comprised of cerebral edema, hemispheric swelling, and diffuse injury [1].
Clinical Presentation
Patients with traumatic parenchymal lesions may present with neurological deterioration, intracranial hypertension, and signs of mass effect on examination or imaging generally with CT [1].
Mortality
Mortality of the different classes of parenchymal lesions correlates with the prognostic variables of TBI in general. Age, postresuscitation GCS, cranial fracture presentation, pupillary response, respiratory insufficiency, ICP, and basal cistern status are factors affecting prognosis [1].
Surgical Treatment
Indication
Patients presenting with GCS between 6 and 8 with frontal or temporal contusions >20 cm3 with MLS >5 mm and patients with lesion > 50 cm3 in volume should be evacuated. Surgical indications, in addition to CT parameters, also need to take into account patients’ clinical status and the occurrence of clinical deterioration [1].
Standard of Care
Current options for surgical treatment of focal parenchymal lesions include craniotomy with evacuation of the lesion. Surgical evacuation of the lesion aids in correcting cerebral shift and reduction of ICP. Study shows that craniotomy, when performed before neurological deterioration in patients, had significantly better outcomes when compared to delayed operation. Stereotactic evacuation of the lesions has also been used for treatment; however, stereotactic treatment is less effective if patient has a diffuse pathology and intracranial hypertension [1].
Latest Advances
Several studies show that decompressive procedures such as hemispheric DC may have a role in management of parenchymal injuries. One study shows that in 28 patients undergoing unilateral or bilateral DC for posttraumatic edema and intracranial hypertension, 57 % showed good outcome or moderate disability at 1 year. In 39 and 18 TBI patients with posttraumatic diffuse brain swelling who underwent primary decompression and secondary decompression respectively, 58 % of the first group and 65 % of the second group showed good outcome or moderate disability at 1 year [1].
In another recent, multicenter decompressive craniectomy (DECRA) trial, study showed that although DC decreased ICP, duration of mechanical ventilation, and time in ICU compared to standard, nonsurgical treatment, patients receiving the craniectomy had lower median score in Extended Glasgow Outcome Scale and resulted in higher risk of unfavorable outcome. This result may be associated with the possibility of the craniectomy allowing expansion of the swollen brain outside the skull, leading to axonal stretch and neural injury [19]. Critics of this trial noted that selection biases affecting randomization, nonstandard criteria for surgical indication and cross-over of subjects may have impacted outcomes.
Nonsurgical Treatment
Indication
Patients with stable ICP, no mass effect on CT scan, and no evidence of neurological deterioration are often managed nonsurgically [1].
Standard of Care
Medical treatment of traumatic parenchymal lesions include protocols involving head elevation, propofol sedation, mannitol and/or hypertonic saline, normocarbia, mild hypothermia to 33–35 °C, and electroencephalogram burst suppression. However, medical management alone shows 3.8 times increase in relative risk of unfavorable outcome compared to DC [1].
Latest Advances
Citicoline, also known as CDP-Choline, has been suggested to aid in both neuroprotection and neuro-restorative process. By increasing levels of glutathione, citicoline showed a reduction in oxidative stress, which reduces the risk of secondary injury in TBI. The Citicoline Brain Injury Treatment (COBRIT) trial tested the effects of 90 days of citicoline on functional outcomes of patients with TBI; however, the results showed that citicoline had no significant improvement compared to placebo as an acute/post acute therapy for TBI patients. 35.4 % of citicoline treated group showed improvement in Extended Glasgow Outcome Scale compared to 35.6 % improvement in the placebo group. The lack of improvement with citicoline treatment may be due to the heterogeneous pathophysiology of TBI and suggested that future drugs target specific subtypes of TBI [20].
Recent study also showed that the standard care of ICP monitoring in TBI patients does not have a significant increase in functional and cognitive status of patients when compared to treatment based on imaging and clinical examination. The 6 month mortality in ICP monitoring patients was 39 % and in imaging and clinical examination, 41 %. Length of stay in ICU in both groups was similar, with 12 days in ICP monitoring and 9 days in imaging-clinical examination. These results suggest that in the treatment of TBI patients, the role of current treatment protocol associated with ICP monitoring and manipulation should be reassessed [21].
Depressed Cranial Fractures
Overview
Incidence
Depressed cranial fracture (DCF) can be present and complicate up to 6 % of head injuries. 90 % of the depressed cranial fractures are comprised of compound cranial fractures [1].
Mortality
Compound cranial fractures have an infection rate of 1.9–10.6 % and an average neurological morbidity of 11 %, incidence of epilepsy of 15 %, and mortality rate of 14–19 % [1].
Clinical Presentation
Patients may present with head pain, swelling, overlying scalp disruption, or any symptoms typically associated with brain injury. Figure 13.3 shows a CT scan of patient with a linear occipital skull fracture.
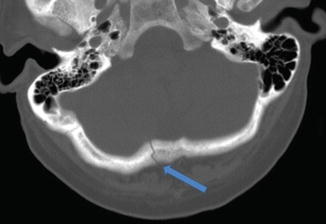

Fig. 13.3
Noncontrast CT scan of linear occipital skull fracture
Surgical Treatment
Indication
Surgery is indicated if fracture depression is greater than the thickness of cranium or as per the surgeon’s judgment. Simple depressed fractures may be managed both surgically and nonsurgically [2].
Standard of Care
Although controversy exists in determination of appropriate surgical treatment of DCF, the conventional surgical treatment of compound DCF involves debridement and surgical elevation. Simple DCF requires surgical elevation only when depression is equal to or greater than the thickness of adjacent intact bone. Reasons for aggressive treatment of DCF involve potential occurrence of infection, epilepsy, and for cosmetic deformity. Study shows that compound cranial fracture patients show 10.6 % incidence of infection and 15 % incidence of late epilepsy [1].
Latest Advances
Traditional treatment of DCF removes bone fragments in order to reduce danger of infection, unstable fixation and intracranial pressure. Recent studies have shown that replacement of the bone fragments did not show a significant increase in risk of infection compared to the removal of bone fragments. By replacing bone fragments in the first operation, second cranioplasty may be obviated. Dissociate bone flap cranioplasty has been shown to provide additional benefits in comparison to lever-up cranioplasty. The advantages of this method is that it avoids epidural hematoma which may form during lever-up craniplasty procedures, can repair dura rupture, treat complicated fractures comminuting across superior sagittal sinus, allow direct vision for surgeons, and avoid secondary operation [22].
Nonsurgical Treatment
Indication
Nonsurgical treatment may be considered if all of the following criteria are met:
No evidence of dural penetration
No significant intracranial hematoma
No frontal sinus involvement
No wound infection or gross contamination
No gross cosmetic deformity [1]
Standard of Care
Antibiotics are used for all compound depressed fractures to prevent infection. Nonsurgical therapy is often reserved for nondisplaced closed fractures. Continuous monitoring of the wound should be done. If significant hematoma does not resolve within 2–3 days, surgery should be performed [1].
Penetrating Intracranial Injury
Overview
Incidence
Penetrating intracranial injury comprises all traumatic brain injuries not resulting from blunt trauma. Of the penetrating injuries, gunshot wound to the head (GSWH) is most common and occurs in 35 % of deaths in brain injury patients and is the proximal cause of the death in greater than 90 % of the victims [2].

Full access? Get Clinical Tree








