2. Aortocaval compression occurs at approximately 20 weeks of gestation when the parturient is positioned supine and the gravid uterus compresses both the aorta and inferior vena cava. This makes it paramount to position the pregnant patient with left uterine displacement (LUD), or lateral position to maintain effective preload [15].
3. Oxygen consumption increases from 40% to 60% from prepregnancy values secondary to the enlarged uterus, the placenta, and fetus.
4. ECG changes are common in pregnancy, given the elevation of the diaphragm by the uterus resulting in a leftward axis. However, depending on the gestational age, there can be an axis deviation in either direction, which is normal. In addition, premature atrial contractions and sinus tachycardia are commonly observed.
C. Respiratory
1. Pregnant women have increased airway mucosal edema secondary to increased blood volume [16], which contributes to the eightfold increase in failed intubation seen in parturients. In addition, there is concern for tissue friability and nasal intervention is discouraged.
3
2. The elevation of the diaphragm as the uterus enlarges results in decreased functional residual capacity (FRC) of up to 40% at term. However, closing capacity does not change; therefore in the supine position, small airway closure and shunting occur resulting in arterial desaturation. During periods of apnea, clinically significant desaturation can occur quickly in the supine position. In a study evaluating desaturation (SaO2 < 90%), after a period of apnea following 99% denitrogenation, desaturation occurred after 4 minutes of apnea for parturients and 7.5 minutes for nonparturients [17].
3. Increased minute ventilation occurs in parturients, likely secondary to progesterone sensitization of the central respiratory centers. This results in an increase in respiratory rate (15%) and tidal volume (40%). Normal CO2 in pregnancy is about 32 mm Hg versus 40 mm Hg in the nonpregnant patient. There is increased renal excretion of sodium bicarbonate to compensate for this hypocapnia, resulting in a minimal increase in pH (7.41 to 7.44) [17].
D. Gastrointestinal
1. Gastric emptying does not decrease throughout pregnancy, but it is slowed with painful contractions and during opioid administration.
2. The placenta produces gastrin, which in theory can result in increased secretions and decreased pH. However, studies have shown that gastrin levels are actually reduced in pregnancy [18].
3. Progesterone and estrogen cause relaxation of smooth muscle tone, including lower esophageal sphincter (LES) tone [19]. Also, the gravid uterus causes rotation of the stomach, resulting in decreased compliance of the LES.
3
4. The risk of aspiration pneumonia is increased in the pregnant patient, given the increased gastric secretions, decreased gastric pH, and compromised LES tone. All pregnant patients undergoing surgery should receive aspiration prophylaxis, including an H2 blocker, promotility agent, and nonparticulate antacid prior to induction of anesthesia, as well as a rapid sequence induction (RSI).
E. Renal
1. Renal blood flow is increased by 75% and GFR is increased by 60% during pregnancy, resulting in a BUN and creatinine decrease by 50% to 60% from prepregnancy values [20].
2. There is a reduction in glucose and bicarbonate tubular reabsorption, possibly resulting in diabetes mellitus (gestational diabetes) and compensatory metabolic acidosis in response to the respiratory alkalosis.
3. Primary peripheral vasodilation causes increased aldosterone levels resulting in increased sodium and water retention. This can cause increased edema in intracranial lesions, leading to worsening of symptoms.
F. Hepatic
1. There is no increase in blood flow to the liver during pregnancy.
2. The clearance of drugs is reduced secondary to the increased volume of distribution associated with the increase in blood volume.
3. There is an increase in the splanchnic, portal, and esophageal venous pressure, which results in esophageal varices in 60% of parturients [20].
4. Serum albumin decreases up to 60% due to an increase in plasma volume [20].
5. Transaminases remain normal, with elevations in alkaline phosphatase, due to increased placental production.
6. During pregnancy, pseudocholinesterase levels are mildly decreased, but no documented cases of prolonged paralysis have been noted following succinylcholine administration [21].
G. Hematologic
1. A dilutional anemia of pregnancy is normal, with the hematocrit decreasing to 30% to 35%.
2. Gestational thrombocytopenia is observed in a small number of parturients (90,000 to 100,000). There is no associated platelet dysfunction or increased risk of bleeding complications.
3. There is an increase in coagulation factors Ι, VII, VIII, and X, a decrease in protein S, and inhibition of fibrinolysis. These changes result in a prothrombic state [22].
III. Uterine blood flow and perfusion
4
A. Uterine blood flow (UBF) is not autoregulated; therefore it is dependent solely on maternal perfusion pressure.
1. UBF is about 700 cc/min (approximately 10% to 15% of cardiac output).
2. Hypovolemia, vasodilators, anesthesia medications, positive pressure ventilation, sympathetic blockade, aortocaval compression, uterine hypertonicity (due to oxytocin or α-adrenergic stimulation) can all cause decreases in UBF.
3. Excessive hyperventilation and hypocapnia should be avoided during general anesthesia in order to prevent placental vasoconstriction and fetal compromise.
B. Vasopressors
4
1. Ephedrine and phenylephrine are both acceptable for use in pregnancy, but phenylephrine has been shown to result in less fetal acidosis and crosses the placenta with less efficacy. It is therefore the vasopressor of choice. Ephedrine appears to stimulate fetal metabolism, resulting in acidosis [23].
2. Vasopressin receptors (V1) are present on the human uterus and administration of vasopressin for hypotension should be avoided, as this can induce uterine contractions [24].
3. Epinephrine is a suitable third-line agent (after phenylephrine and ephedrine) for treatment of hemodynamic instability. Epinephrine will cross the placenta and can cause a dose-dependent decrease in UBF from vasoconstriction. In addition, epinephrine has an effect on β-adrenergic receptors. As a tocolytic, it can be used to decrease uterine contractions [25].
IV. Pharmacology
A. Placental transfer of water and solutes
1. Movement is dependent on hydrostatic, osmotic pressure gradients, and concentration gradients.
2. Transporter-mediated transport and endocytosis occur at the plasma membrane and may be faster than simple diffusion.
3. The higher the lipid solubility, the increased ease of transfer across the placenta.
4. Molecular weight can limit transfer. Five hundred Daltons seem to be the upper limit in order for complete placental transfer.
5. The maternal cytochrome P450 system is induced during pregnancy, resulting in a decreased fraction of free drug [26].
B. Placental transfer of anesthesia drugs
1. Inhalational agents freely cross the placenta, are of low molecular weight and extremely lipophilic.
2. Opioids freely cross the placenta, are lipophilic and of low molecular weight.
3. Induction agents (propofol, thiopental, etomidate) also freely cross the placenta, but first pass maternal hepatic metabolism reduces fetal exposure.
4. Neuromuscular blocking and reversal agents are highly ionized making placental transfer minimal.
5. Additional medications
a. Anticholinergics. Glycopyrrolate has minimal transfer due to ionization resulting from its quaternary ammonium structure. Atropine and scopolamine freely pass as they are minimally ionized and are tertiary amines.
b. Anticoagulation. Coumadin (<500 Daltons) crosses the placenta freely as it is minimally ionized and small. Heparin is ionized and does not cross the placenta. If the parturient has taken coumadin, the fetus is anticoagulated as well. Unfortunately, there is not a mechanism for fetal reversal of this anticoagulation until after delivery.
c. Antihypertensives. All β-blockers, hydralazine, nitroprusside, and nitroglycerin cross the placenta. It should be noted that β-blockers can cause a transient fetal bradycardia, but may be necessary to decrease maternal blood pressure or heart rate. Angiotensin-converting enzyme (ACE) inhibitors are contraindicated in the second and third trimesters secondary to teratogenic effects [27], so they are not typically used.
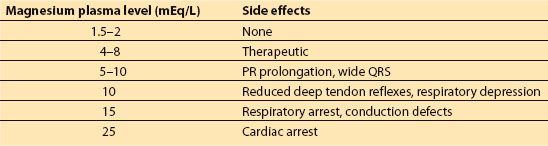
d. Magnesium sulfate is used for seizure prophylaxis in preeclampsia and for neuroprotection. This medication can cause hypotension, uterine atony, and hypotonia. The side effects of magnesium are listed in Table 25.2.
Table 25.2 Magnesium toxcity
e. Mannitol is used in neurosurgical procedures to assist with reduction of ICP. However, this drug accumulates in the fetus, resulting in a hyperosmolar state, reduced urinary blood flow and lung fluid production. Individual case reports demonstrate that doses of 0.25 to 0.5 mg/kg appear to be safe [28].
V. Fetal effects
A. Medications
5
1. All inhalational agents have been shown to have neuroprotective effects in adult animal models and are used in neurosurgical anesthesia cases; however, they must be administered at less than 1 MAC (preferably at <0.7 MAC) to reduce cerebral vasodilation and minimize increases in ICP. Isoflurane or sevoflurane is recommended for neurosurgical procedures because they also reduce cerebral metabolic rate and have a lesser effect on ICP. In rodent neonatal models, when compared to sevoflurane and isoflurane, desflurane resulted in increased neuroapoptosis, so there may be a reason to avoid this inhalational agent in parturients [29].
2. Benzodiazepines do not appear to cause any teratogenic effects [30], even when used in the first trimester.
3. Nitrous oxide is a weak teratogen in rodents [31,32]. Even though nitrous oxide inhibits methionine synthase (and therefore DNA synthesis), neurologic symptoms cannot be corrected with the coadministration of folic acid. The etiology of the teratogenicity is likely multifactorial and its use is not recommended during pregnancy.
5
4. When mannitol is administered, there is concern for fetal dehydration (oligohydramnios), increased osmolarity and electrolyte concentrations. Individual case reports show that doses of 0.25 to 0.5 mg/kg appear to be safe [28].
5. β-blockers cross the placenta and can place the fetus at risk for fetal bradycardia, as well as neonatal bradycardia, hypoglycemia, and respiratory depression [33]. Atenolol and metoprolol cross the placenta more readily than labetolol (100% vs. 40% respectively). Although there is minimal placental transfer of esmolol (20%), there have been reports of profound fetal bradycardia requiring emergency cesarean delivery [34]. β-blockers are useful to blunt the hemodynamic changes of intubation and extubation; therefore labetalol is the best option for the parturient.
6. Steroids are routinely used in neurosurgery to decrease the perifocal edema seen with intracranial mass lesions. The use of dexamethasone or betamethasone has decreased the risk of respiratory distress syndrome, intraventricular hemorrhage, and neonatal death in premature infants. However, recent studies have shown that repeat doses of antenatal steroids over time have resulted in decreased placental size and neonatal birth weight [35]. The use of steroids for maternal benefit during neurosurgical procedures is probably not harmful though and may decrease neonatal morbidity and mortality if the fetus is delivered within several days of the surgical intervention.
7. All anticonvulsants cross the placenta and have the potential to cause neural tube, orofacial, cardiovascular and digital malformations, as well as fetal coagulopathies. Valproate, phenytoin, carbamazepine, phenobarbital, and topiramate all have been associated with congenital malformations [36]. Most structural abnormalities will occur with use in the first trimester; however, the research is focused on chronic use and little is known about acute use of anticonvulsive agents. Levetiracetam (Keppra) is commonly used in neurosurgical procedures, and in one recent study from Australia, there were no malformations seen in parturients taking Keppra [37].
8. Hyperventilation is frequently used during neurosurgery to reduce ICP; however, excessive hypocapnia can result in uteroplacental vasoconstriction increasing the risk for fetal compromise (ETCO2 < 25).
B. Radiation exposure
1. Ionizing radiation exposure could result in spontaneous abortion, congenital malformation, or fetal cerebral injury depending on the dose and the timing of exposure. Exposure during the first 15 weeks of development places the fetus at greatest risk for injury and declines by a factor of 4 after 15 weeks of gestation, likely due to organogenesis. After 26 weeks of gestation, the risks are minimal. The American College of Obstetricians and Gynecologists (ACOG) recommend that exposure not exceed 5 rad (radiation absorbed dose). The parturient should be shielded with lead in the anterior and posterior position during any exposure to minimize risk. For reference, a computed tomography (CT) of the chest results in fetal exposure of up to 0.1 rad and a chest x-ray results in exposure of <0.001 rad [38].
2. Radiopaque agents used in CT scans can contain iodine and cross the placenta, potentially resulting in fetal hypothyroidism. These agents should be used if the benefit outweighs the risk to the fetus [39].
3. The safety of paramagnetic contrast agents (gadolinium) has not been established in humans, but they do cross the placenta and should be used only if the benefit outweighs the risk [39].
VI. Anesthetic considerations specific to the parturient
A. General considerations
1. For all anesthetic procedures, all pregnant patients should receive a nonparticulate acid, an H2 blocker, and a gastric motility agent.
2. An rapid sequence induction is recommended.
3. When the gestational age is >20 weeks, left uterine displacement should be maintained.
4. Parturients should wear sequential compression devices when nonambulatory, given the hypercoagulable state of pregnancy.
5. Agents that increase ICP should be avoided, as in any craniotomy for the nonpregnant patient (e.g., ketamine). Succinylcholine may produce transient increases in ICP. However, those increases may be abolished with IV lidocaine, adequate depth of anesthesia, hyperventilation, or a defasciculating dose of a nondepolarizing paralytic [40].
B. Blood pressure monitoring
1. In such cases, an arterial line should be utilized. The blood pressure should be maintained within 20% of baseline, with a mean arterial pressure of >70 mm Hg. During aneurysm clipping, there may be a need to induce hypotension acutely, but this should be avoided if possible.
2. Sodium nitroprusside can be used to lower blood pressure, but it crosses the placenta and may cause fetal cyanide toxicity (keep infusions to less than 0.5 mg/kg/h).
3. Nitroglycerin can also be used to control blood pressure without adverse fetal effects, although experimentally, nitroglycerin is metabolized to nitrites, causing methemoglobinemia.
4. Inhalation agents like isoflurane can also be used to lower blood pressure and even at greater than 1 MAC, uteroplacental perfusion is maintained as long as maternal blood pressure is maintained [41].
C. Fetal monitoring. ACOG recommends the following:
1. Any fetus termed viable (approximately 24 weeks) should have minimum Doppler fetal heart tones and tocometer analysis immediately prior to and after an operation. Continuous intraoperative monitoring should be performed if (1) there is an obstetrical physician or nurse qualified to interpret the fetal tracing; (2) monitoring is possible during the surgery; and (3) an emergency cesarean delivery could be performed without compromising the parturient’s safety. Neurosurgery should take place in a facility that has obstetrical, pediatric, and neonatal expertise readily available. The operating room should be equipped for an emergency cesarean section should the need arise [42].
2. Any fetus termed previable should have Doppler confirmation of fetal heart rate prior to and immediately after the surgical procedure. Continuous intraoperative fetal monitoring for a previable fetus should be performed to optimize placental blood flow in cases of fetal bradycardia and on a case-by-case basis [42].
3. Blood pressure should be maintained within 20% of maternal baseline and should be increased if fetal bradycardia ensues.
4. Loss of beat-to-beat variability is normal during general anesthesia. However in awake patients, it can be a sign of fetal compromise. Fetal heart rate decelerations are abnormal and should be corrected with increased blood pressure, increased oxygenation, or a change in position.
D. Tocolytics. Tocolytics may be needed if uterine contractions are detected on the tocometer during surgery.
1. Inhalational agents are potent tocolytics, but can cause increases in ICP at MAC greater than 0.7 to 1, so their use in neurosurgical procedures is limited.
2. Calcium channel blockers (nicardipine and nifedipine) and intravenous hydration are frequently used to prevent preterm contractions. However, calcium channel blockers can cause hypotension. Hydration must be balanced with the maternal risk of cerebral edema.
3. Alternatives are terbutaline and NTG, but one should be aware that both of these can result in maternal hypotension. In addition, terbutaline can result in maternal and fetal tachycardia as well as maternal pulmonary edema.
4. Magnesium has not been shown to prevent preterm labor when compared with placebo, but has shown benefit in fetal neuroprotection [43].
VII. Anesthetic induction
A. Induction agents
6
1. We recommend using either thiopental 5 to 7 mg/kg (if available), or propofol 2 mg/kg as induction agents, as both may have neuroprotective effects in the setting of mild cerebral ischemia [44]. It should be noted that these medications do not appear to have neuroprotective effects in situations of severe ischemia in humans. Ketamine is controversial because of its potential effects on ICP and should be used with caution [45]. In addition, ketamine has been associated with increased fetal neuroapoptosis in the rhesus monkey model. However, any animal data should be interpreted with caution when extrapolating to human subjects [46]. Etomidate has been favored in the past for its hemodynamic stability when used as an induction agent. However, it has been associated with adrenal suppression in trauma and critically ill patients, even in one dose [47].
2. Opioids readily cross the placenta and are appropriate for induction to blunt the hemodynamic response of laryngoscopy. However if a cesarean is performed, the neonatal team should be aware of the fetal exposure and likely respiratory depression. Remifentanil is an acceptable option for use during intubation and throughout the case. Studies have shown that neonates delivered to parturients who have received remifentanil have minimal respiratory depression that is usually self-limited [48].
3. All parturients should undergo rapid sequence intubation using either rocuronium 1.2 mg/
kg or succinylcholine 1 mg/kg.
4. Parturients have increased airway edema, weight gain, increased breast tissue, and decreased FRC, making intubation more difficult. Studies have suggested that the incidence of failed intubation in the obstetric population is approximately 1:300, which is eight times higher than that of the general surgical population [49]. Emergency airway equipment should be immediately available for such cases, given the increased risk of failed intubation. In addition, we suggest “ramping” the patient to align the external auditory meatus with the sternal notch, in order to align the oral, pharyngeal, and laryngeal axes to improve intubating conditions.
VIII. Anesthetic maintenance
A. Total intravenous anesthesia (TIVA) versus inhalational agents. TIVA is preferred if neuromonitoring will be performed during the surgery since inhalational agents can result in decreased amplitude and increased latency of the electrical signals recorded. If inhalational agents are used, sevoflurane and isoflurane may be preferred over desflurane for reasons discussed above. Inhalational agents should be maintained at no more than 0.7 MAC in order to minimize increases in cerebral blood flow and thus reduce ICP. Also, remember MAC is reduced by 30% to 40% in parturients.
B. Paralytics. There are no fetal effects, as these drugs do not cross the placenta. Any paralytic agent can be utilized for maintenance of paralysis during the surgical intervention, unless there are maternal contraindications.
C. Glucose management. Maintaining euglycemia intraoperatively is beneficial to both the mother and the fetus. The fetus is dependent on maternal glucose for metabolic oxidative processes and fetal glucose levels are usually about 20 mg/dL less than maternal levels. Fetal metabolic derangements will occur if the fetus becomes hypo- or hyperglycemic, and can result in cardiac dysrhythmias and seizures. Maternal glucose levels are important as well, as hypo- or hyperglycemia in neurosurgery has been associated with adverse outcomes, including increased mortality. The optimal glucose concentration is difficult to determine, but in general, glucose levels of 110 to 150 mg/dL are recommended.
IX. Anesthetic emergence
A. General considerations. It is paramount on emergence of most neurosurgical procedures (for pregnant and nonpregnant patients) that (1) the patient awakens quickly for neurologic assessment; (2) hemodynamic alternations are minimized; and (3) a smooth extubation performed to limit increases in ICP or stress on the cranial vasculature. In addition, the patient must meet the criteria for extubation, including following commands, full reversal from paralytics, adequate oxygenation, and hemodynamic stability. In the parturient, these should be balanced with attempts to maintain adequate placental perfusion as dictated by fetal monitoring and maternal baseline blood pressure. We recommend elevating the head of bed (or using reverse Trendelenburg) during emergence to minimize increases in ICP, as well as decreasing the effects of the gravid uterus on the thoracic cavity.
B. Pharmacologic interventions. Smooth emergence can be accomplished using several pharmacologic interventions (antisympathetic and antinociceptive) listed below.
1. Lidocaine is utilized topically or intravenously as an antinociceptive agent to prevent straining and coughing on the endotracheal tube. Lidocaine is not known to have any teratogenic effects.
2. β-blockers are useful in blunting the hemodynamic changes seen on extubation. See discussion above for details, but labetalol is the β-blocker of choice in pregnancy.
3. Venodilators such as NTG, nitroprusside, and hydralazine all cross the placenta easily and can cause uterine hypoperfusion if systemic blood pressure is too low. NTG and nitroprusside can be useful in reducing elevated blood pressures and are easily titratable, given their short half-lives. Hydralazine has a half-life of several hours and is not as easily titratable.
X. Breastfeeding. The current recommendation from the American Academy of Pediatrics is exclusive breastfeeding for approximately the first 6 months of life. The mother should be encouraged to breastfeed her infant prior to surgical intervention and the mother may express and store her breast milk in the possibility that she is unable to breastfeed postoperatively. After general anesthesia, current opinion recommends breastfeeding as soon as the patient is physically and mentally able to do so, as there does not appear to be infant complications from the standard medications used in a single maternal anesthetic. However, production of breast milk may be decreased after pituitary intervention if the neurohypophysis axis is disrupted, decreasing the production of oxytocin.
XI. Conclusion. Pregnancy creates unique challenges for the anesthesiologist due to the physiologic changes seen in pregnancy and concern for fetal well-being. Intracranial pathologies do not appear to occur more frequently in pregnancy, but these patients can be at higher risk for hemorrhage and growth due to the physiologic changes in pregnancy. Maintaining hemodynamic stability is critical as UBF is not autoregulated, and dependent on maternal blood pressure. Most anesthetic drugs appear safe in pregnancy; however, animal models have shown possible teratogenic effects. Fetal monitoring should be utilized based on gestational age and availability of surgical intervention.
REFERENCES
1. Bateman BT, Olbrecht VA, Berman MF, et al. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology. 2012;116:324–333.
2. Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27:855–865; discussion 865–866.
3. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798.
4. Moodley J. Maternal deaths due to hypertensive disorders in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2008;22:559–567.
5. Tuluc M, Brown D, Goldman B. Lethal vertebral artery dissection in pregnancy: a case report and review of the literature. Arch Pathol Lab Med. 2006;130:533–535.
6. Wisoff JH, Kratzert KJ, Handwerker SM, et al. Pregnancy in patients with cerebrospinal fluid shunts: report of a series and review of the literature. Neurosurgery. 1991;29:827–831.
7. Ikossi DG, Lazar AA, Morabito D, et al. Profile of mothers at risk: an analysis of injury and pregnancy loss in 1,195 trauma patients. J Am Coll Surg. 2005;200:49–56.
8. Haas JF, Janisch W, Staneczek W. Newly diagnosed primary intracranial neoplasms in pregnant women: a population-based assessment. J Neurol Neurosurg Psychiatry. 1986;49:874–880.
9. Lee LS, Chi CW, Chang TJ, et al. Steroid hormone receptors in meningiomas of Chinese patients. Neurosurgery. 1989;25:541–545.
10. Chan MT, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782–786.
11. Popitz-Bergez FA, Leeson S, Thalhammer JG, et al. Intraneural lidocaine uptake compared with analgesic differences between pregnant and nonpregnant rats. Reg Anesth. 1997;22:363–371.
12. Hirabayashi Y, Shimizu R, Saitoh K, et al. Acid-base state of cerebrospinal fluid during pregnancy and its effect on spread of spinal anaesthesia. Br J Anaesth. 1996;77:352–355.
13. Lund CJ, Donovan JC. Blood volume during pregnancy. Significance of plasma and red cell volumes. Am J Obstet Gynecol. 1967;98:394–403.
14. Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–399.
15. Bamber JH, Dresner M. Aortocaval compression in pregnancy: the effect of changing the degree and direction of lateral tilt on maternal cardiac output. Anesth Analg. 2003;97:256–258.
16. Kodali BS, Chandrasekhar S, Bulich LN, et al. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–362.
17. McClelland SH, Bogod DG, Hardman JG. Apnoea in pregnancy: an investigation using physiological modelling. Anaesthesia. 2008;63:264–269.
18. Murray FA, Erskine JP, Fielding J. Gastric secretion in pregnancy. J Obstet Gynaecol Br Emp. 1957;64:373–381.
19. Ulmsten U, Sundstrom G. Esophageal manometry in pregnant and nonpregnant women. Am J Obstet Gynecol. 1978;132:260–264.
20. Paech MJ, Scott K. Liver and renal disease. In: Obstetric Anesthesia and Uncommon Disorders. Gambling D, Douglas MJ, McKay RSF, eds. Cambridge: Cambridge University Press; 2008:249–257.
21. Blitt CD, Petty WC, Alberternst EE, et al. Correlation of plasma cholinesterase activity and duration of action of succinylcholine during pregnancy. Anesth Analg. 1977;56:78–83.
22. Stirling Y, Woolf L, North WR, et al. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–182.
23. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
24. Maggi M, Del Carlo P, Fantoni G, et al. Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J Clin Endocrinol Metab. 1990;70:1142–1154.
25. Segal S, Csavoy AN, Datta S. The tocolytic effect of catecholamines in the gravid rat uterus. Anesth Analg. 1998;87:864–869.
26. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008.
27. Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451.
28. Tuncali B, Aksun M, Katircioglu K, et al. Intraoperative fetal heart rate monitoring during emergency neurosurgery in a parturient. J Anesth. 2006;20:40–43.
29. Kodama M, Satoh Y, Otsubo Y, et al. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991.
30. Wikner BN, Kallen B. Are hypnotic benzodiazepine receptor agonists teratogenic in humans? J Clin Psychopharmacol. 2011;31:356–359.
31. Mazze RI, Fujinaga M, Rice SA, et al. Reproductive and teratogenic effects of nitrous oxide, halothane, isoflurane, and enflurane in Sprague-Dawley rats. Anesthesiology. 1986;64:339–344.
32. Fujinaga M, Mazze RI, Baden JM, et al. Rat whole embryo culture: an in vitro model for testing nitrous oxide teratogenicity. Anesthesiology. 1988;69:401–404.
33. Witter FR, King TM, Blake DA. Adverse effects of cardiovascular drug therapy on the fetus and neonate. Obstet Gynecol. 1981;58:100S–105S.
34. Ducey JP, Knape KG. Maternal esmolol administration resulting in fetal distress and cesarean section in a term pregnancy. Anesthesiology. 1992;77:829–832.
35. Sawady J, Mercer BM, Wapner RJ, et al. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Beneficial Effects of Antenatal Repeated Steroids study: impact of repeated doses of antenatal corticosteroids on placental growth and histologic findings. Am J Obstet Gynecol. 2007;197:281. e281–e288.
36. Werler MM, Ahrens KA, Bosco JL, et al. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann Epidemiol. 2011;21:842–850.
37. Vajda FJ, Graham J, Roten A, et al. Teratogenicity of the newer antiepileptic drugs–the Australian experience. J Clin Neurosci. Official Journal of the Neurosurgical Society of Australasia. 2012;19:57–59.
38. Groen RS, Bae JY, Lim KJ. Fear of the unknown: ionizing radiation exposure during pregnancy. Am J Obstet Gynecol. 2012;206:456–462.
39. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647–651.
40. Kovarik WD, Mayberg TS, Lam AM, et al. Succinylcholine does not change intracranial pressure, cerebral blood flow velocity, or the electroencephalogram in patients with neurologic injury. Anesth Analg. 1994;78:469–473.
41. Dahlgren G, Tornberg DC, Pregner K, et al. Four cases of the ex utero intrapartum treatment (EXIT) procedure: anesthetic implications. Int J Obstet Anesth. 2004;13:178–182.
42. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 474: Nonobstetric surgery during pregnancy. Obstet Gynecol. 2011;117:420–421.
43. King JF. Tocolysis and preterm labour. Curr Opin Obstet Gynecol. 2004;16:459–463.
44. Schifilliti D, Grasso G, Conti A, et al. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs. 2010;24:893–907.
45. Mayberg TS, Lam AM, Matta BF, et al. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anesthesia in patients undergoing craniotomy. Anesth Analg. 1995;81:84–89.
46. Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384.
47. Hildreth AN, Mejia VA, Maxwell RA, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65:573–579.
48. Ngan Kee WD, Khaw KS, Ma KC, et al. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104:14–20.
49. Barnardo PD, Jenkins JG. Failed tracheal intubation in obstetrics: a 6-year review in a UK region. Anaesthesia. 2000;55:690–694.

Full access? Get Clinical Tree







