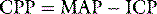Chapter 102 Neurological emergencies in children
Neurological emergencies are the most common life-threatening emergencies in children. In developed societies after the first year of life, the leading cause of death in childhood is injury, particularly traumatic brain injury. There is a range of conditions affecting the brain, spinal cord and peripheral nervous system that require prompt recognition, resuscitation and definitive management. The pathophysiology, clinical features, treatment and outcome of these acute neurological emergencies are influenced by several important differences between adults and children. These differences include response to injury, developmental maturity and capacity for growth and recovery.
PATHOPHYSIOLOGY OF BRAIN INJURIES IN CHILDREN
Features of brain injury particular to the paediatric patient are described below.
BONE DEVELOPMENT
The skull bones in the first year of life are thin with open sutures and open fontanelles. Beyond 2 years, the skull sutures close and the cranial vault thickens. In young children there tends to be less bony protection from high impact trauma, while the non-rigid skull may expand to partially decompress expanding lesions.1
UNDIAGNOSED COMA
An ordered approach to diagnosis and treatment is required for a child with depressed conscious state of unknown origin. This approach must consider common life-threatening and rare treatable diseases (Table 102.1).
Table 102.1 Causes of coma in children
| Structural | Metabolic |
|---|---|
| Trauma | Post-ictal state |
| Accidental | Infection |
| Inflicted | Meningitis |
| Hydrocephalus | Encephalitis |
| Haemorrhage | Drugs and toxins |
| AVM | Hypoxia–ischaemia |
| Aneurysms | Circulatory shock |
| Tumour | Biochemical |
| Tumour | Hypoglycaemia |
| Cerebral abscess | Electrolyte disorders |
| Sodium/water | |
| Calcium | |
| Acid–base disturbance | |
| Hyperthermia | |
| Hepatic failure | |
| Haemolytic–uraemic syndrome | |
| Inborn errors of metabolism | |
| Reye’s syndrome |
INITIAL MANAGEMENT
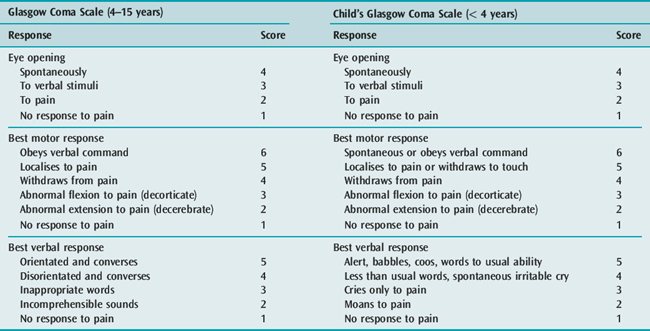
Concurrent with the initial assessment and resuscitation, relevant details of the present and past history should be obtained. A detailed neurological and general physical examination should be performed. It is important to document accurately the conscious state so that changes over time, particularly deterioration, can be easily recognised. The Glasgow Coma Scale (GCS) is appropriate for this purpose. The responses of children change with development and therefore the GCS requires modification for paediatric use (Table 102.2). After completing the clinical assessment, the likely diagnosis is often apparent and appropriate investigation and treatment can commence. Multiple factors may compound to produce coma. For example, a child with severe gastroenteritis may have hyperthermia, hyponatraemic dehydration, metabolic acidosis and hypovolaemic shock.
CONTROLLED VENTILATION
Indications for ventilating a comatose child are:
Once ventilation is initiated the stomach should be drained with a gastric tube and blood pressure checked every 5 minutes. Raised ICP should be considered in any case of rapidly progressive coma. Intracranial hypertension should be managed with moderate hyperventilation and intravenous mannitol (0.25 g/kg). Hypertonic saline given as 0.5 ml/kg of 20% solution (3.4 mmol/ml) can also rapidly reduce ICP.2 Once stability is achieved, adequate sedation and analgesia is required. Muscle relaxants may be necessary to facilitate ventilation and prevent straining; however, their use precludes further neurological assessment and therefore, if long-acting muscle relaxants are continued, ICP monitoring is advisable. Hyperventilation is a short-term manoeuvre and following the early resuscitation phase, gradual return to a low-normal PaCO2 should be the aim. This is best achieved with end tidal CO2 and ICP monitoring. Particular attention should be paid to restoring intravascular volume and maintaining an adequate CPP.
ADDITIONAL INVESTIGATIONS
Additional investigations include arterial blood gas analysis, serum electrolytes, glucose, urea and creatinine, liver function tests, serum ammonia, serum and CSF lactate and pyruvate, and urine analysis. Appropriate screening of blood and urine will exclude common poisons and drug intoxications.
STATUS EPILEPTICUS
Convulsive status epilepticus (CSE) is usually defined as a continuous convulsion lasting 30 minutes or longer or repeated convulsions lasting 30 minutes or longer without recovery of consciousness between convulsions.3 The common causes of CSE in children are:
PATHOPHYSIOLOGY
Many physiological changes occur during prolonged seizures. There is an initial phase of compensation lasting less than 30 minutes. Following a period of transition there is a phase of decompensation commencing between 30 and 60 minutes and evolving over hours. Physiological changes during the compensated phase include tachycardia, hypertension, increased catecholamine release and increased cardiac output. Changes within the brain include increased cerebral blood flow and increased cerebral utilisation of glucose and oxygen. After 30–60 minutes the mechanisms for homeostatic compensation fail. During the decompensated phase there may be falling blood pressure and cardiac output, hypoglycaemia, hypoxia, acidosis, electrolyte disturbance and rhabdomyolysis. The cerebral physiology is characterised by failing autoregulation and reduced cerebral blood flow and oxygen and glucose utilisation. Over hours a deficit in brain energy develops and this is associated with the development of brain damage.4
EXCITATORY AMINO ACIDS AND BRAIN INJURY
Mesial temporal sclerosis is the most common acquired brain lesion following CSE. There is evidence that the accumulation of a number of excitatory and inhibitory amino acids have a role in the pathophysiology of neuronal injury. In particular, glutamate accumulation and stimulation of NMDA receptors (N-methyl-D-aspartate) leads to an influx of intracellular calcium, which triggers a number of cytotoxic events and ultimately cell death.5
MANAGEMENT
The initial management is as for other neurological emergencies with attention to airway and oxygenation. Most seizures in childhood cease spontaneously in a short time, but if they persist for 5 minutes or continue after presentation to an emergency department, they should be stopped to avoid metabolic and ischaemic neuronal damage. Hypoglycaemia should be excluded or detected early. If intravenous access cannot be obtained rapidly, drugs can be administered intramuscularly, intranasally, rectally or via the intraosseous (i.o.) route. If benzodiazepines are administered within 20 minutes of a seizure commencing, the rate of seizure control is higher than if they are administered after 30 minutes.6 This justifies early prehospital administration of benzodiazepines to children with active seizures at the time of ambulance arrival.7 Specific drug treatment includes the following.
BENZODIAZEPINES
Diazepam, midazolam and lorazepam are the most useful agents.
Lorazepam (0.05–0.1 mg/kg i.v.) may have advantages over diazepam as it is as effective, has a longer half-life, and causes less respiratory depression.8,9
Midazolam and clonazepam are also effective. When i.v. access is not available, intramuscular or intranasal administration of midazolam is an alternative to rectal diazepam.10–12 In children presenting to an emergency department still convulsing, 0.2 mg/kg i.m. of midazolam has been reported to control 83% of seizures within 5 minutes of administration and 93% of seizures within 10 minutes.13 Midazolam has also been used by constant i.v. infusion (1–8 μg/kg per min) to control refractory CSE in children.14–16
PHENYTOIN
Phenytoin should be commenced if benzodiazepines are not effective. It is given as 20 mg/kg i.v. over 30 minutes, which is followed by a maintenance dose (4 mg/kg 8-hourly past the neonatal period). It causes minimal sedation or respiratory depression, but is not suitable in neonates and in patients who are already on maintenance doses. As it is given slowly, phenytoin will not have rapid effects and if generalised CSE persists, consideration should be given to the use of thiopental (see below). Fosphenytoin, a prodrug of phenytoin, can be administered i.m. or i.v., but has a less irritant effect when given i.v.17 Its place in the management of CSE in children is not yet clear.18
PARALDEHYDE
In many centres rectal paraldehyde (0.4 ml/kg mixed with an equal quantity of olive oil) is frequently used in the management of CSE.18 One advantage of paraldehyde is that, like diazepam, it can be administered rectally if i.v. access is difficult to obtain.
OUTCOME
The outcome of CSE is dependent on the aetiology. Neurologically normal children in whom CSE is precipitated by fever are considered to have a good prognosis with mortality reported between 0 and 2%.19 The incidence of neurological deficits or cognitive impairment in this group is also very low.5 In acute symptomatic CSE (where CSE is a symptom of an acute neurological process such as infection or trauma), mortality is 12–16% and the incidence of new neurological dysfunction is more than 20%.19 In this setting, however, it is very difficult to tease out the extent to which prolonged seizures contribute to neurological sequelae.
BACTERIAL MENINGITIS
INVESTIGATIONS
Lumbar puncture for CSF microscopy, culture and bacterial antigen testing is required for definitive diagnosis of BM and to guide antibiotic therapy. While an LP can be performed safely in the majority of children with BM, LP may precipitate brainstem herniation if raised ICP is present.20 Identifying children at risk of this complication is difficult, so it is generally recommended that empiric antibiotic therapy be commenced and LP deferred if any of the following clinical features are present:
A normal CT scan does not exclude the possibility of elevated ICP.20 Therefore, the decision to defer an LP should be based on clinical rather than radiological signs. In addition to signs of increased ICP, other indications for deferring the LP include cardiorespiratory instability and severe coagulopathy.
If LP is deferred, alternative methods of establishing a bacterial diagnosis include blood culture and bacterial antigen testing in urine and polymerase chain reaction (PCR) testing of blood.21,22
MANAGEMENT
ANTIBIOTIC THERAPY
Empiric broad-spectrum antibiotics should be selected based on likely pathogens and local resistance patterns. A common protocol for BM is to use ampicillin plus cefotaxime for the first month of life and to use a third-generation cephalosporin (cefotaxime or ceftriaxone) after the first month.23 In regions where penicillin and cephalosporin-resistant Pneumococcus occurs, vancomycin should be added to the initial empiric antibiotics until the causative organism is identified and the antibiotic sensitivities are known. When cephalosporin-resistant pneumococci are found to be the causative organism in meningitis, both a third-generation cephalosporin and vancomycin should be continued as vancomycin penetrates into CSF poorly and therefore should not be used as a single antibiotic. The addition of rifampicin should be considered.24
ADJUVANT THERAPY
Although a number of adjuvant therapies have been investigated experimentally, the only one that is commonly used clinically is dexamethasone. If dexamethasone is used it should ideally be given before the first dose of antibiotics and continued for 48 hours (0.4 mg/kg 12-hourly).25 There is evidence that dexamethasone reduces the incidence of neurological sequelae and sensineural deafness; however, the beneficial effects are greatest in Hib meningitis.26,27 Now that immunisation has changed the epidemiology and antibiotic-resistant pneumococcal strains are more common, it is possible that the relationship between risk and benefit of dexamethasone therapy has changed. However, current recommendations support the use of dexamethasone for children more than 6 weeks of age with bacterial meningitis.24,28

Full access? Get Clinical Tree